Publicado in Henry Rzepa's Blog
Autor Henry Rzepa
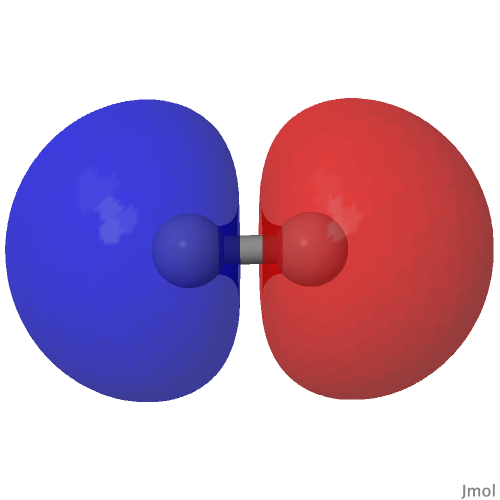
From the last few posts here, you might have noticed much discussion about how the element carbon might sustain a quadruple bond. The original post on this topic from some years ago showed the molecular orbitals of the species CN + , which included two bonding π-types and a low lying nodeless bonding σ-orbital, all with double occupancies and adding up to a triple bond.