Ciencias QuímicasInglés
Publicado in Corin Wagen
Autor Corin Wagen
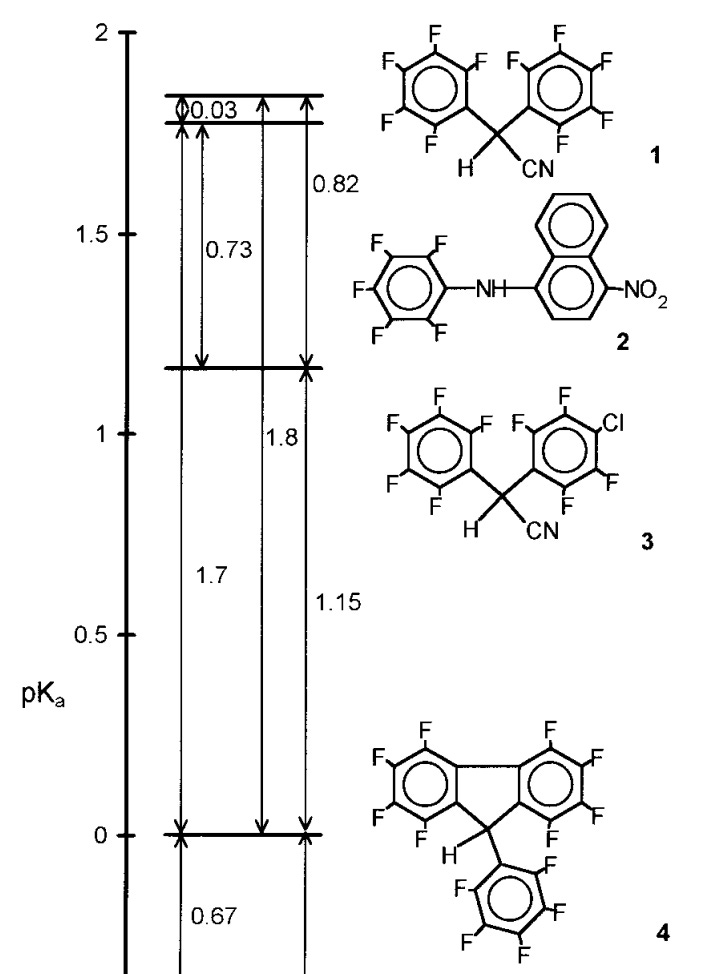
The concept of p K a is introduced so early in the organic chemistry curriculum that it’s easy to overlook what a remarkable idea it is. Briefly, for the non-chemists reading this: p K* a is defined as the negative base-10 logarithm of the acidity constant of a given acid H–A:* p K a := -log 10 ([HA]/[A-][H+]) Unlike pH, which describes the acidity of a bulk solution,