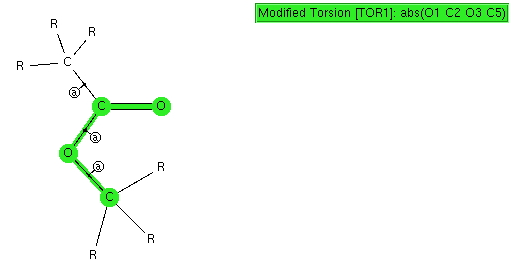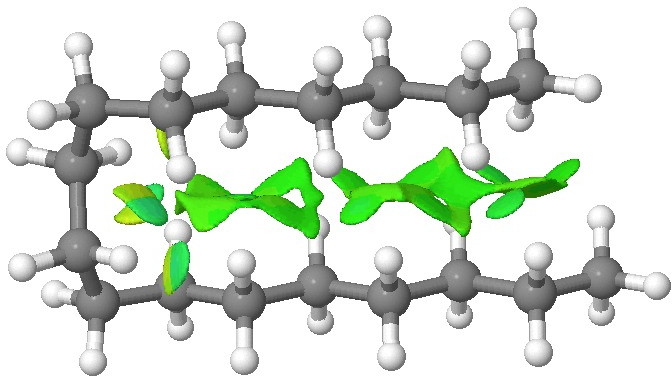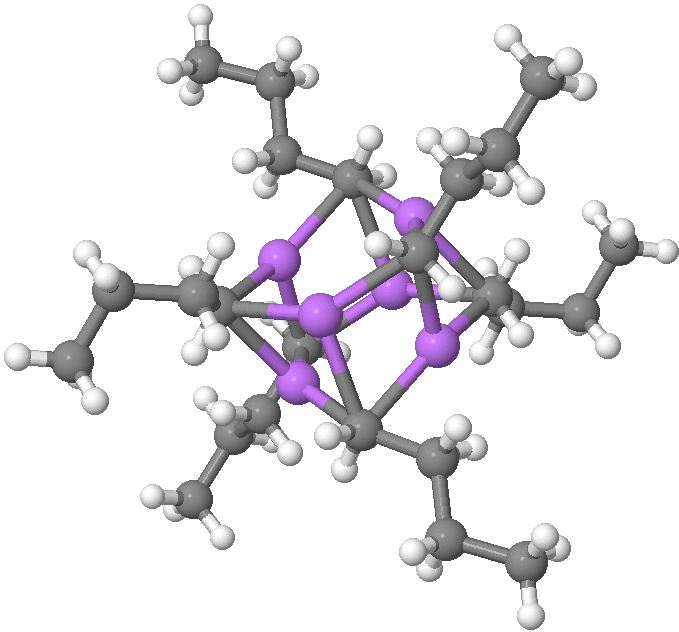
n-Butyl lithium is hexameric in the solid state[cite]10.1002/anie.199305801[/cite] and in cyclohexane solutions. Why? Here I try to find out some of its secrets. SUHBEC. CLICK FOR 3D. The crystal structure reveals the following points of interest: Six lithium atoms form a cluster with triangular faces. An off-centre carbanion caps a triangular lithium face.