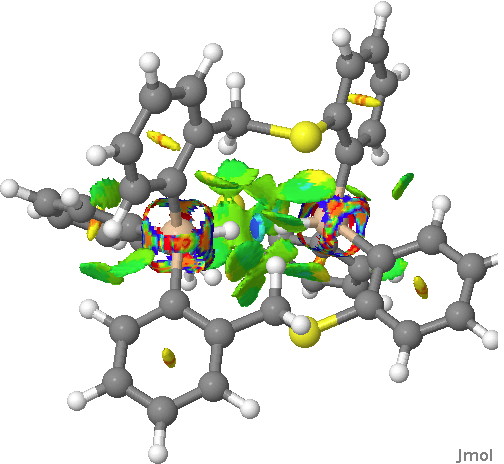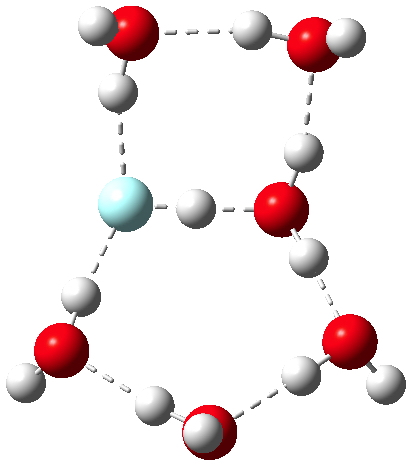
No doubt answers to the question posed in the previous post are already being obtained by experiment. Just in case that does not emerge in the next day or so, I offer a prediction here. The methodology is the same as before, and I have not tried to look for new isomeric forms compared with the structures found with HCl.