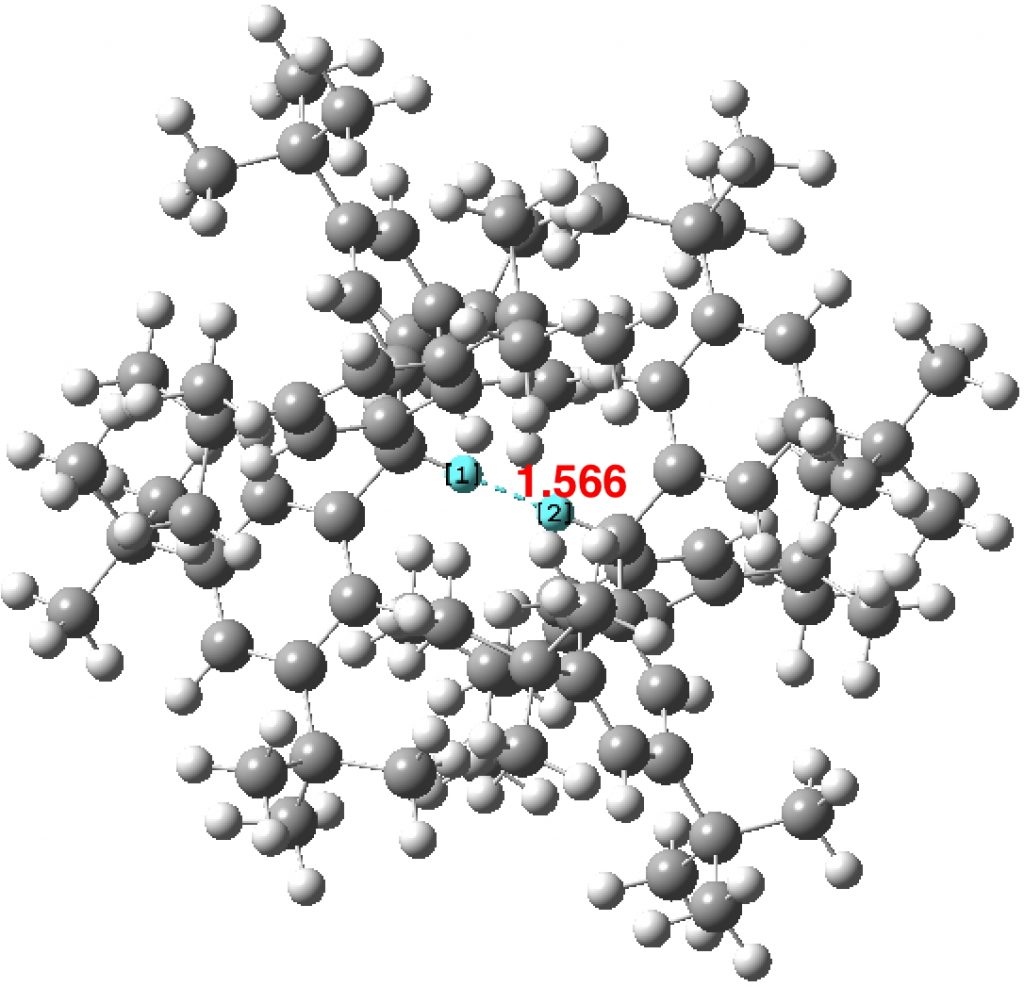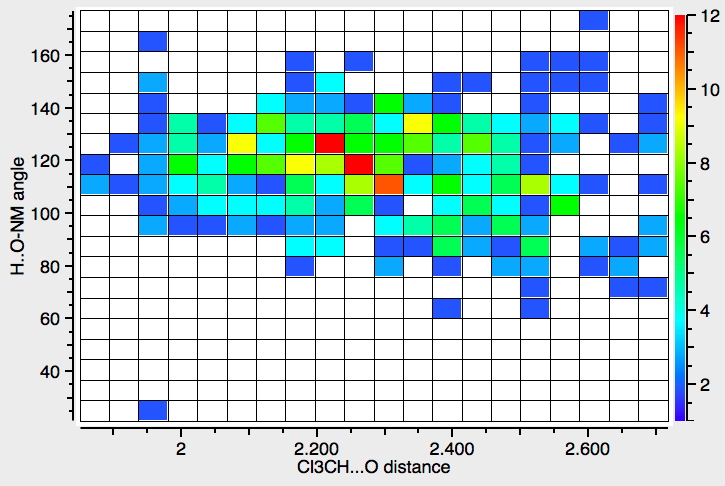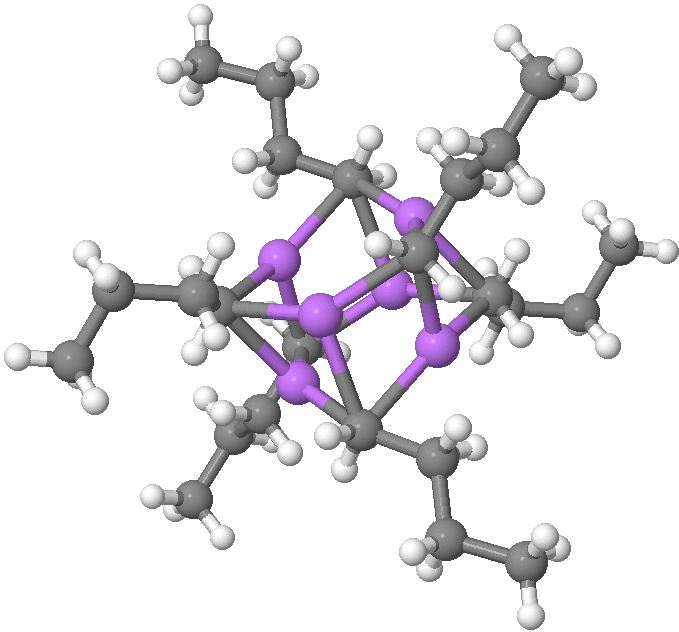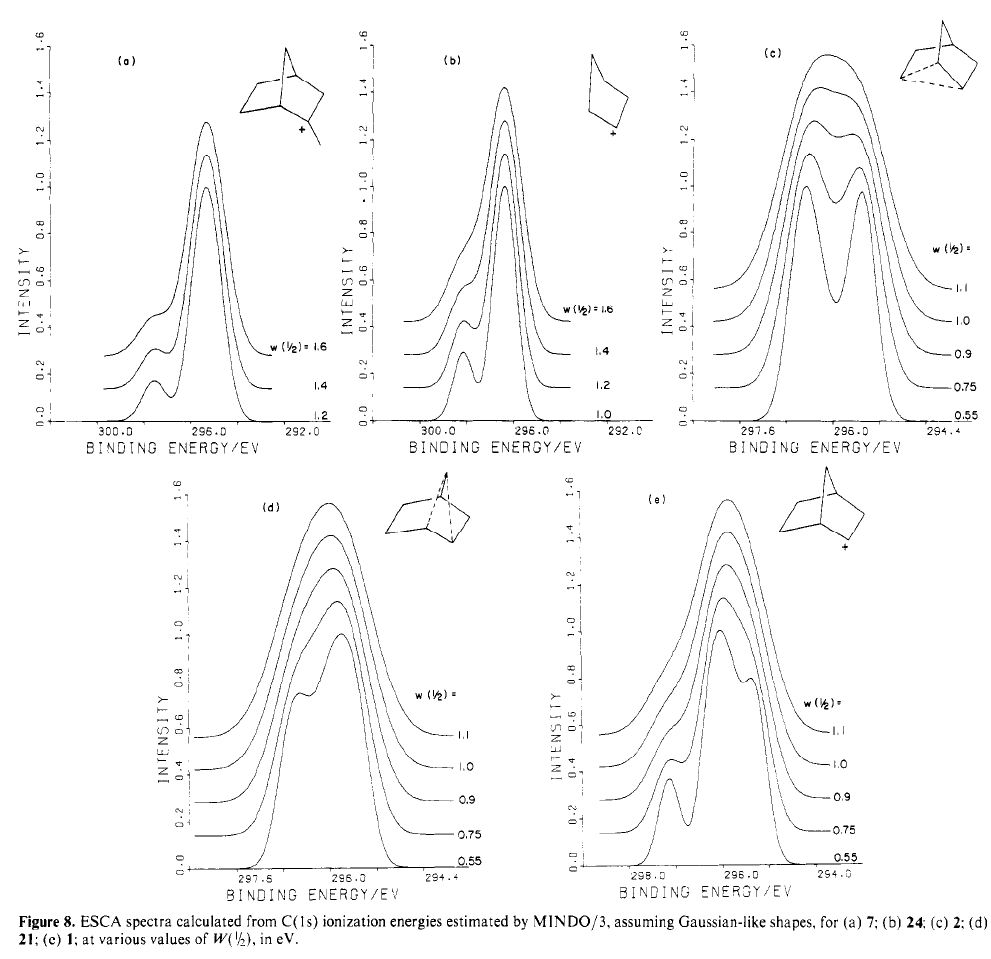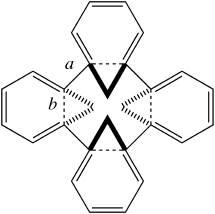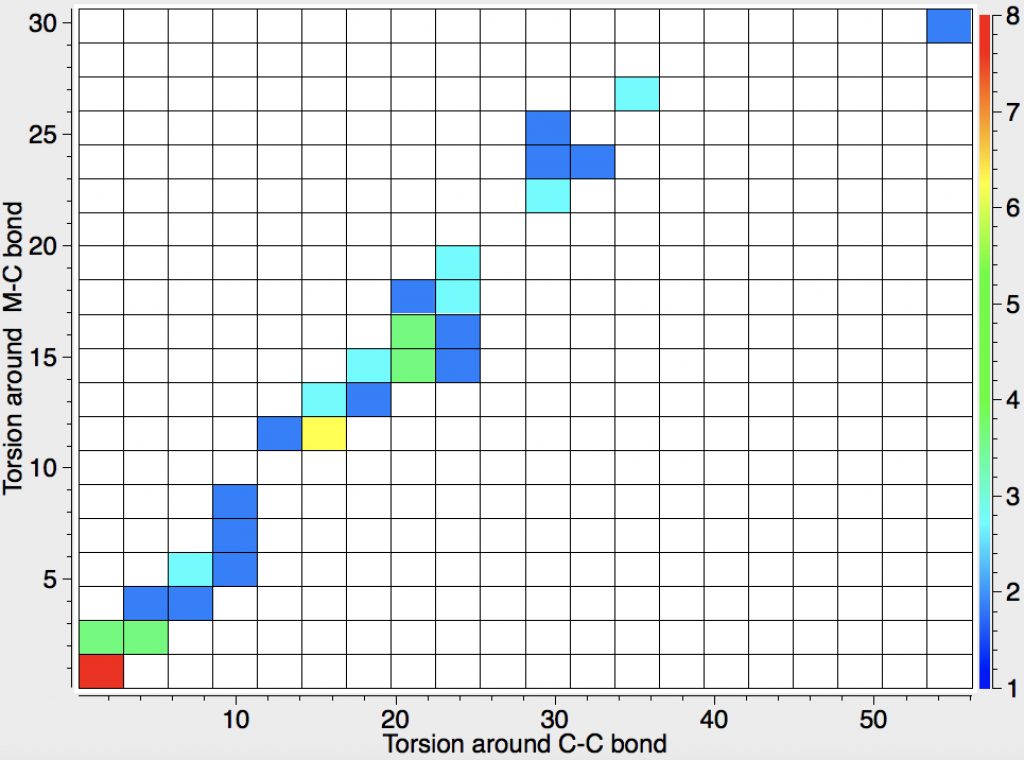
Another selection (based on my interests, I have to repeat) from WATOC 2017 in Munich. Odile Eisenstein gave a talk about predicted 13 C chemical shifts in transition metal (and often transient) complexes, with the focus on metallacyclobutanes. These calculations include full spin-orbit/relativistic corrections, essential when the carbon is attached to an even slightly relativistic element.