A little while ago, I set out some interpretations of how to push curly arrows.
Publicaciones de Rogue Scholar

Many moons ago, when I was a young(ish) lecturer, and much closer in time to my laboratory roots of organic synthesis, I made some chemistry videos. One of these has resurfaced, somewhat (to me at least) unexpectedly. Nowadays of course, such demonstrations are all carried out using virtual simulations (Flash animations etc) as the equipment itself becomes less common.
This is a follow-up to comment posted by Ryan, who asked about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC): “In Van Leusen, it (the isocyanide) acts as an electrophile”. The Wikipedia article (recently updated by myself) shows nucleophilic attack by an oxy-anion on the carbon of the C≡N group, with the isocyanide group acting as the acceptor of these electrons (in other words, the electrophile). In the form shown
Here is another example gleaned from that Woodward essay of 1967 ( Chem. Soc. Special Publications (Aromaticity), 1967 , 21 , 217-249), where all might not be what it seems. Woodward notes that the reaction between the (highly reactive) 1 does not occur.
The previous post described how the acid catalysed ring opening of propene epoxide by an alcohol (methanol) is preceded by pre-protonation of the epoxide oxygen to form a “hidden intermediate” on the concerted intrinsic reaction pathway to ring opening.
I mentioned in the last post that one can try to predict the outcome of electrophilic aromatic substitution by approximating the properties of the transition state from those of either the reactant or the (presumed Wheland) intermediate by invoking Hammond’s postulate[cite]10.1021/ja01607a027[/cite]. A third option is readily available nowadays; calculate the transition state directly. Here are the results of exploring this third variation.
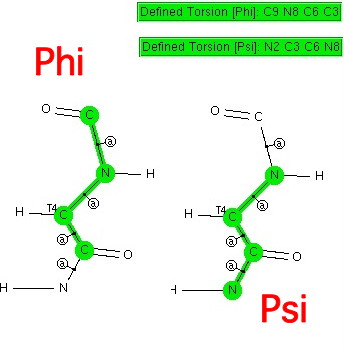
This is really just a postscript to the previous post. There I showed how a search of the (small molecule) crystal database revealed the s-cis conformation about the N-C amide bond (the one with partial double bond character that prevents rotation) and how this conformation means that a C-H approaches quite closely to an adjacent oxygen.
In a previous post, I set out how to show how one can reduce a 1 H NMR spectrum to the structure [A] below. I speculated how a further test could be applied to this structure; back predicting its spectrum using just quantum mechanics. Overkill I know, but how well might the two match? The process must start by considering the conformational possibilities of [A]. Each will have a different predicted spectrum.
It is always rewarding when one comes across a problem in chemistry that can be solved using a continuous stream of rules and logical inferences from them. The example below[cite]10.1039/P19930000299[/cite] is one I have been using as a tutor in organic chemistry for a few years now, and I share it here. It takes around 50 minutes to unravel with students.
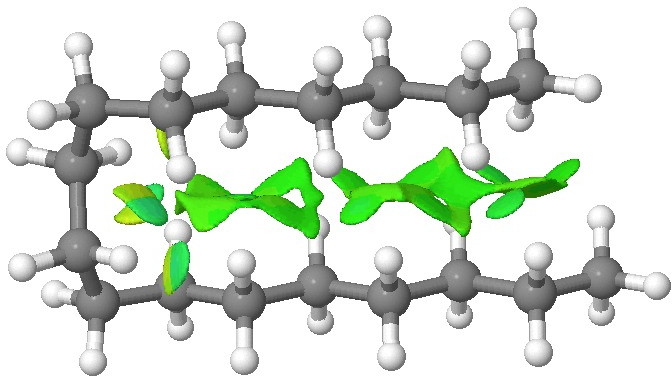
We tend to think of simple hydrocarbons as relatively inert and un-interesting molecules. However, a recent article[cite]10.1002/anie.201202894[/cite], which was in fact highlighted by Steve Bachrach on his blog , asks what “ The Last Globally Stable Extended Alkane ” might be. In other words, at what stage does a straight-chain hydrocarbon fold back upon itself, and no significant population of the linear form remain?