Publié in Henry Rzepa's Blog
Auteur Henry Rzepa
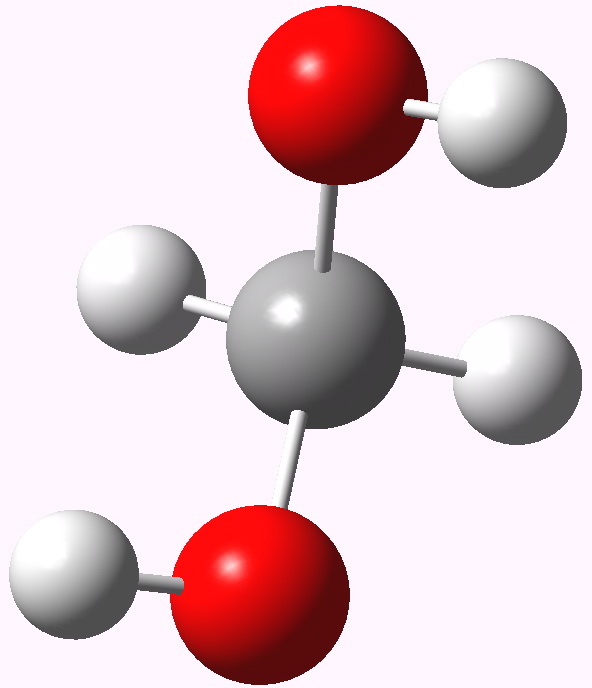
In my previous post I speculated why bis (trifluoromethyl) ketone tends to fully form a hydrate when dissolved in water, but acetone does not. Here I turn to asking why formaldehyde is also 80% converted to methanediol in water? Could it be that again, the diol is somehow preferentially stabilised compared to the carbonyl precursor and if so, why? Methanediol. The lowest energy geometry is shown above.