Publié in Henry Rzepa's Blog
Auteur Henry Rzepa
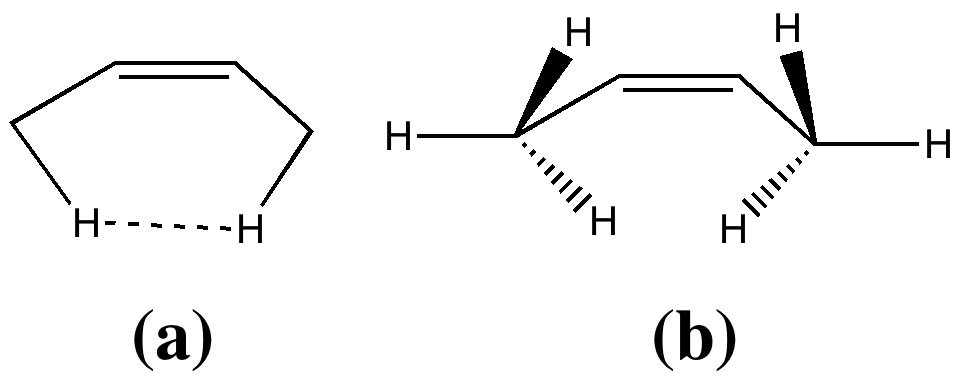
In two previous posts, I have looked at why cis -butene adopts conformation (a) rather than (b). I suggested it boiled down to electronic interactions between the methyl groups and the central alkene resulting in the formation of a H…H “ topological ” bond, rather than attraction between the H…H region to form a weak chemical “ bond “. Here I take a look at what happens when that central C=C bond is gradually removed.