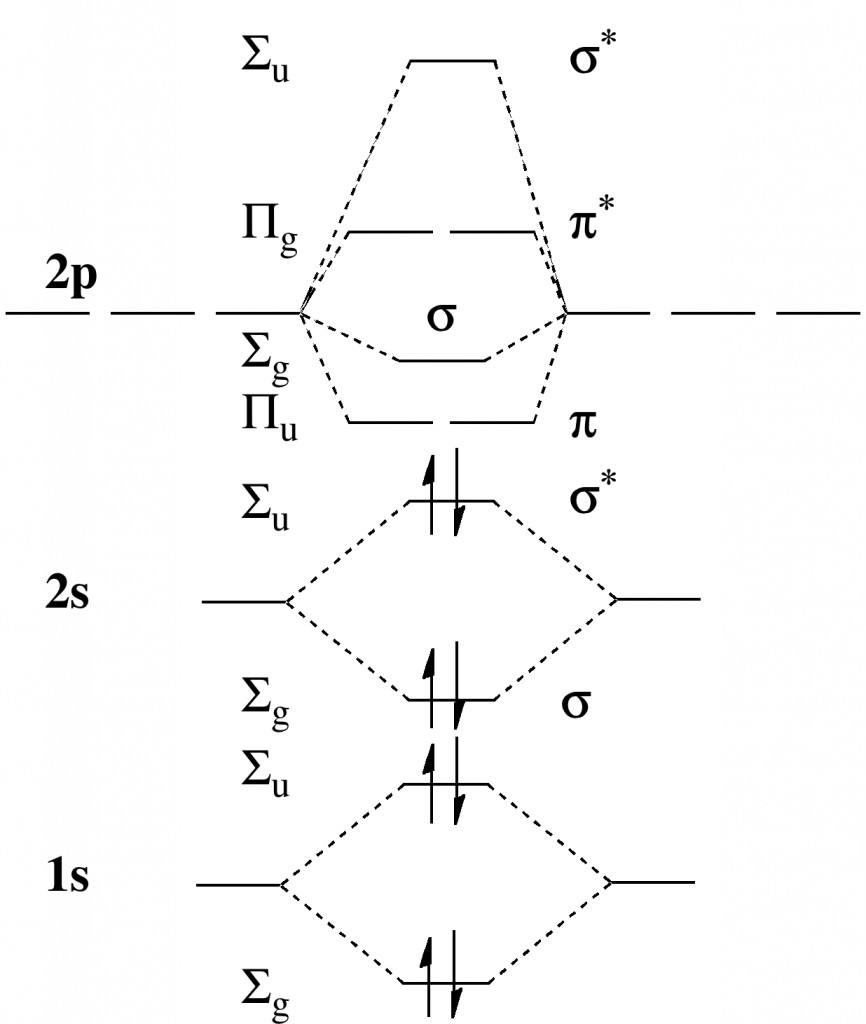
Ever since the concept of a shared two-electron bond was conjured by Gilbert N. Lewis in 1916,[cite]10.1021/ja02261a002[/cite] chemists have been fascinated by the related concept of a bond order (the number of such bonds that two atoms can participate in, however a bond is defined) and pushing it ever higher for pairs of like-atoms.