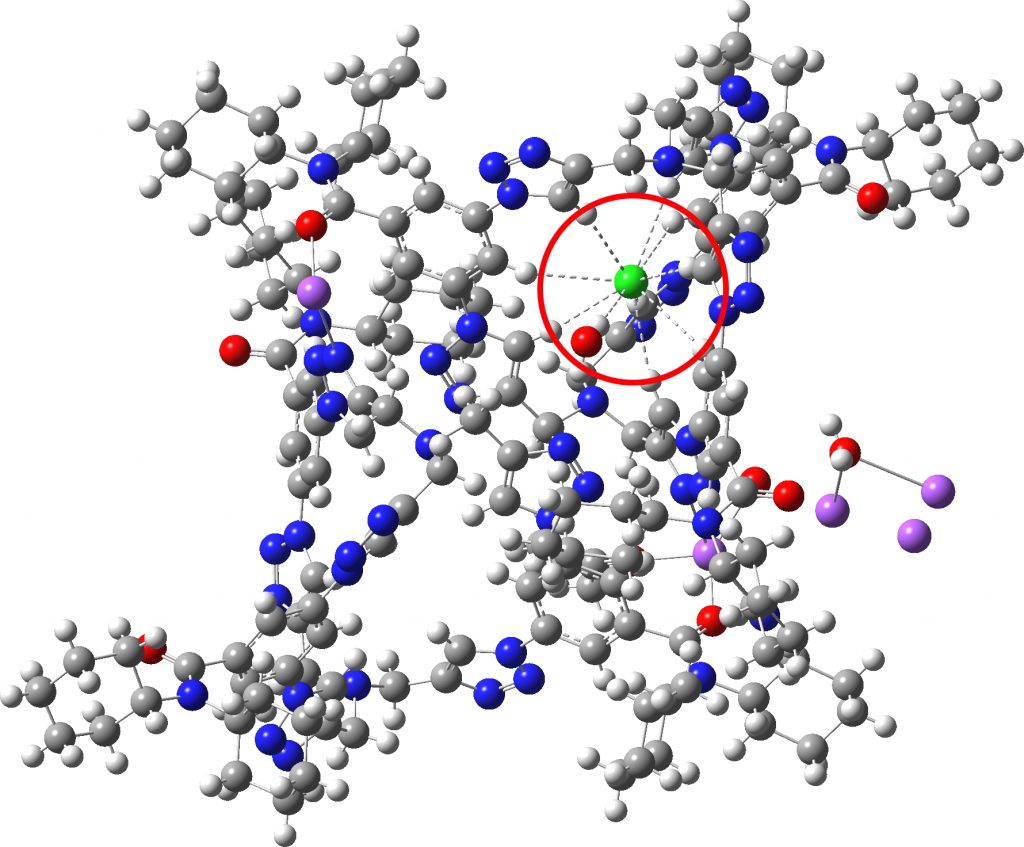
Each year, C&E News runs a poll for their “ Molecule of the year “. I occasionally comment with some aspect of one of the molecules that catches my eye (I have already written about cyclo[18]carbon, another in the list). Here, it is the Incredible chloride cage , a cryptand-like container with an attomolar (10 17 M -1 ) affinity for a chloride anion.[cite]10.1126/science.aaw5145[/cite] The essence of