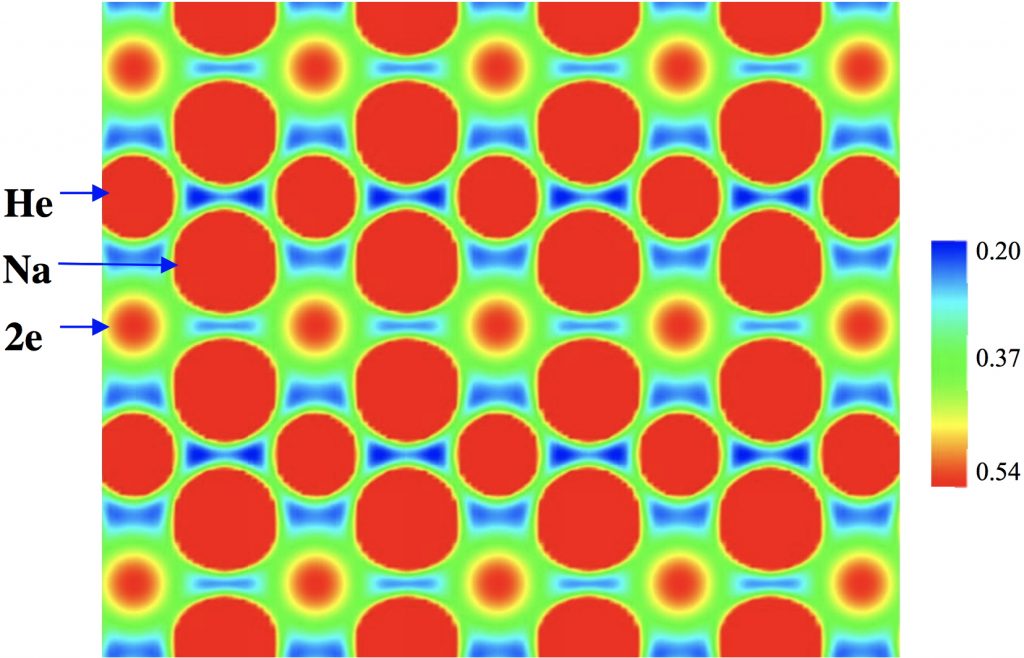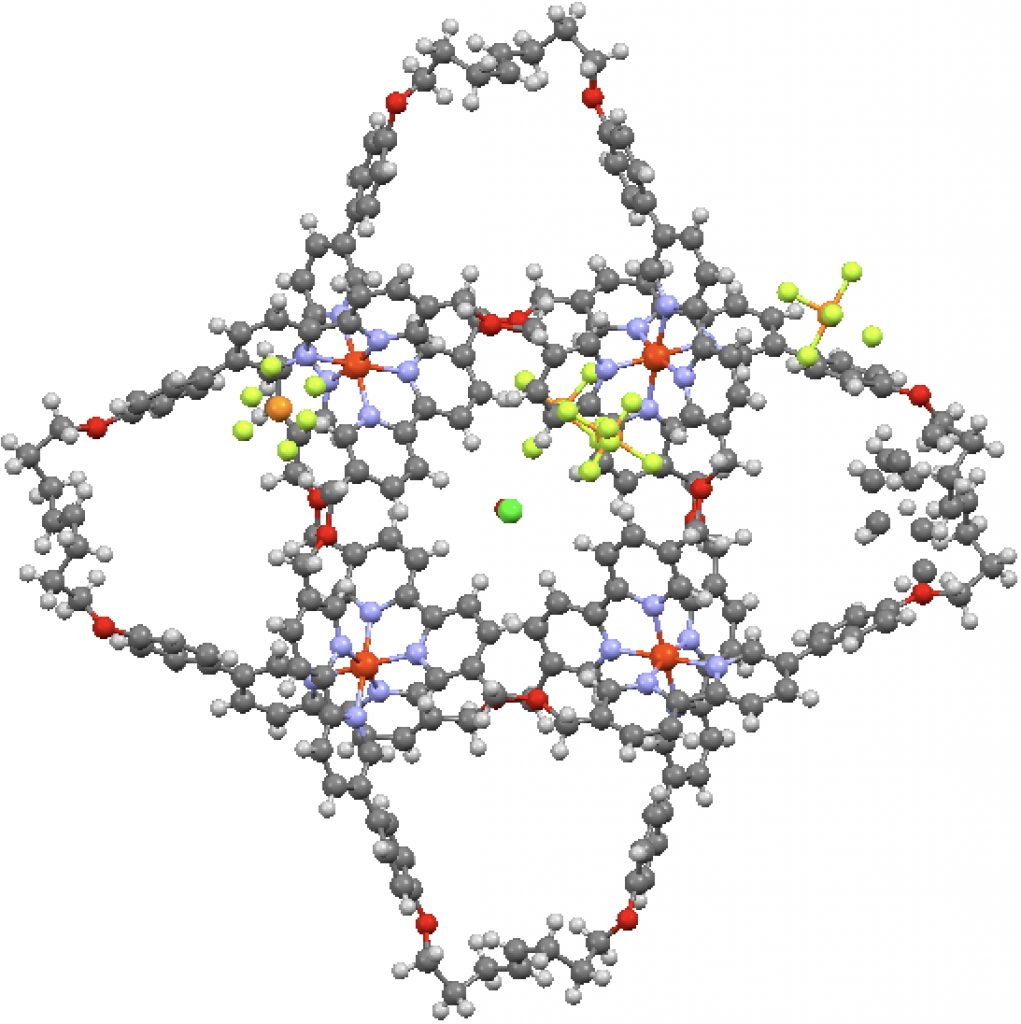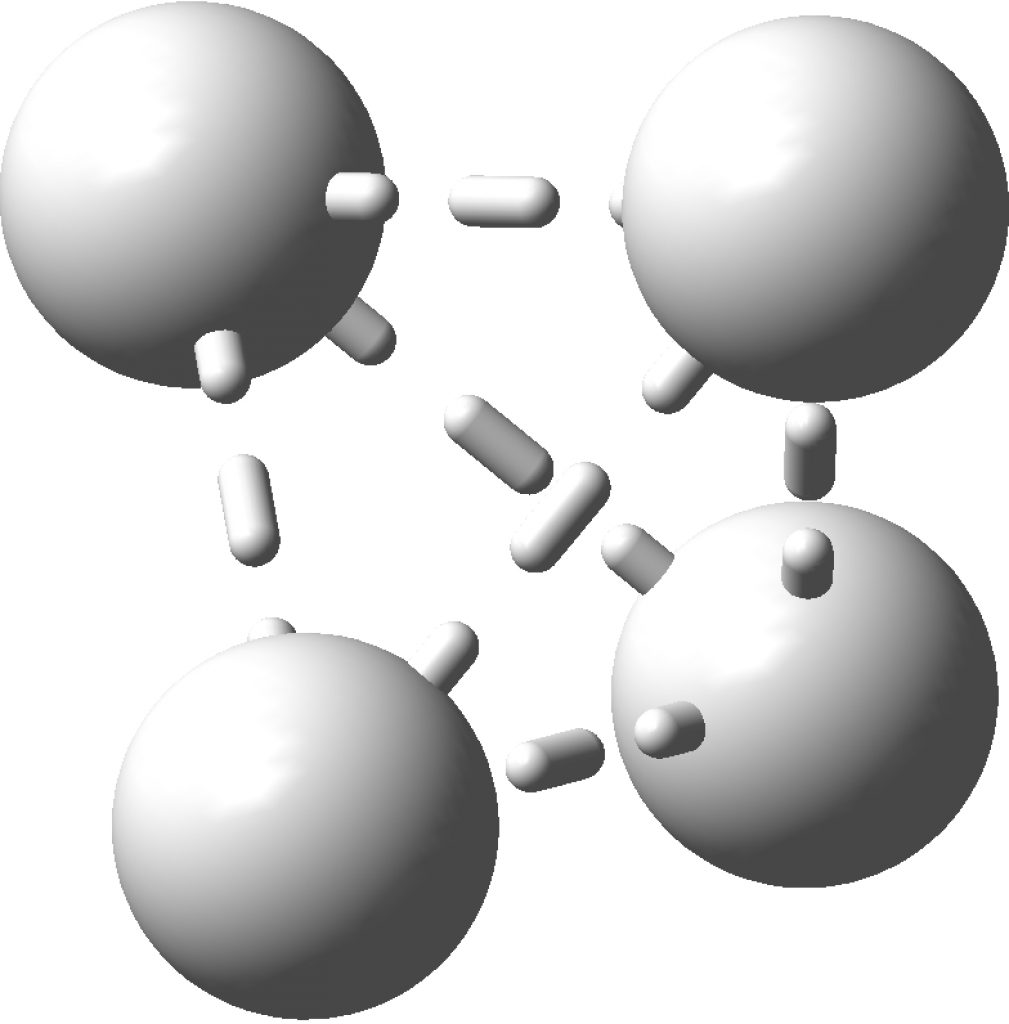
This post arose from a comment attached to the post on Na 2 He and relating to peculiar and rare topological features of the electron density in molecules called non-nuclear attractors. This set me thinking about other molecules that might exhibit this and one of these is shown below.