Henry Armstrong studied at the Royal College of Chemistry from 1865-7 and spent his subsequent career as an organic chemist at the Central College of the Imperial college of Science and technology until he retired in 1912.

Henry Armstrong studied at the Royal College of Chemistry from 1865-7 and spent his subsequent career as an organic chemist at the Central College of the Imperial college of Science and technology until he retired in 1912.
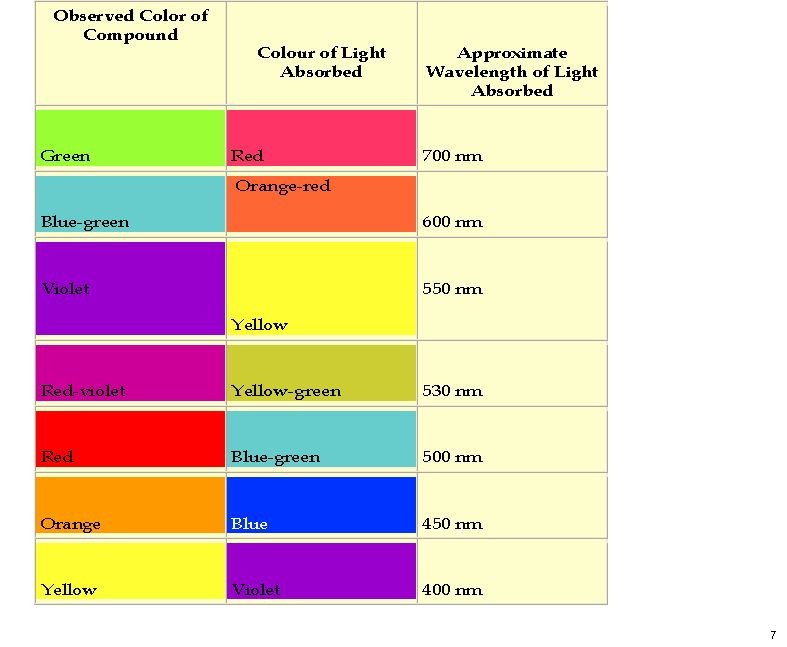
My previous post on the topic of mauveine left the outcome dangling. Put simply, λ max is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve. Can the theoretical prediction be out by 110nm, or might it be the structure of the molecule itself that has been wrongly described?
As the title hints, I have been here before. The S N 1 solvolysis mechanism of t-butyl chloride was central to the flourishing of physical organic chemistry from the 1920s onwards, and it appears early on in most introductory lecture courses and text books. There we teach that it is a two-stage mechanism.
Introductory organic chemistry invariably features the mechanism of haloalkane solvolysis, and introduces both the Sn1 two-step mechanism, and the Sn2 one step mechanism to students. They are taught to balance electronic effects (the stabilization of carbocations) against steric effects in order to predict which mechanism prevails.
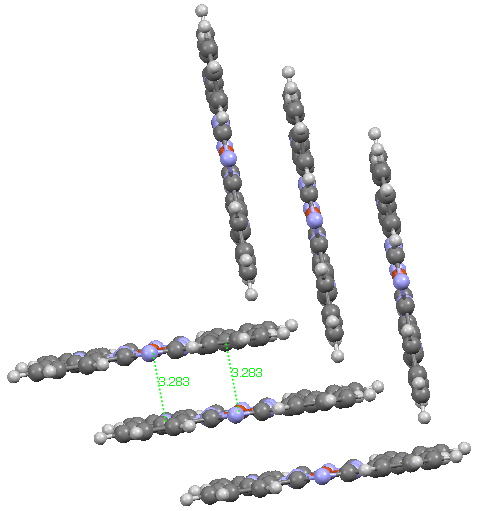
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λ max 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.
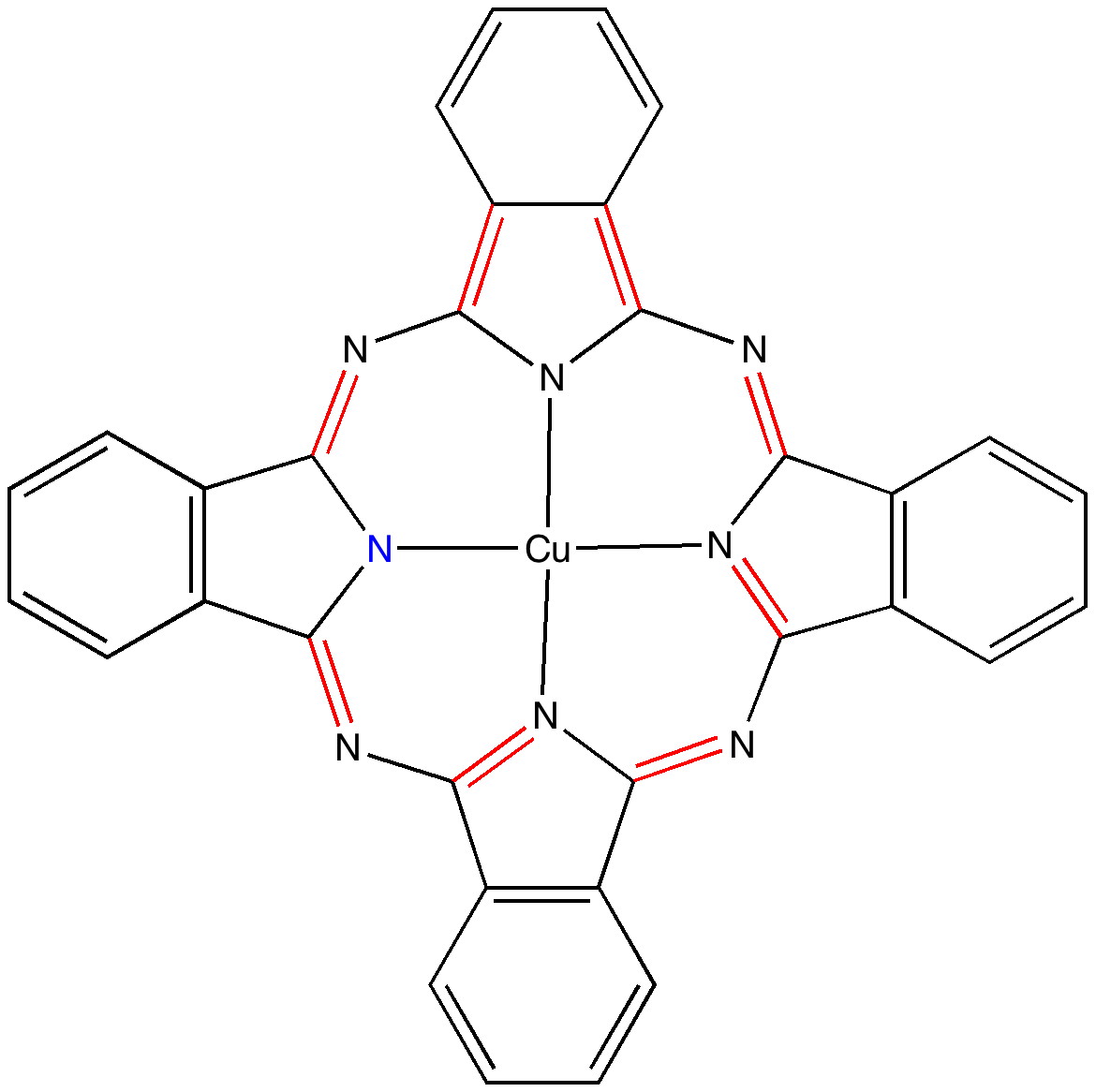
The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else.
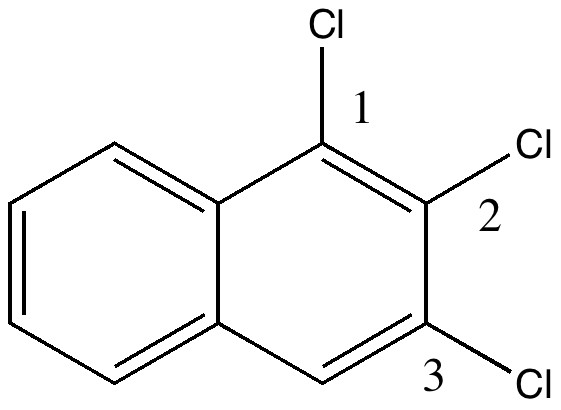
In 1890, chemists had to work hard to find out what the structures of their molecules were, given they had no access to the plethora of modern techniques we are used to in 2010. For example, how could they be sure what the structure of naphthalene was? Well, two such chemists, William Henry Armstrong (1847-1937) and his student William Palmer Wynne (1861-1950;

The molecule below was characterised in 1996 (DOI: 10.1246/cl.1996.489) and given the name tris(dithiolene)vanadium (IV). No attempt was made in the original article to give this molecule a Lewis structure using Lewis electron pair bonds. This blog will explore some of the issues that arise when this is attempted. 1 NAMPOG.

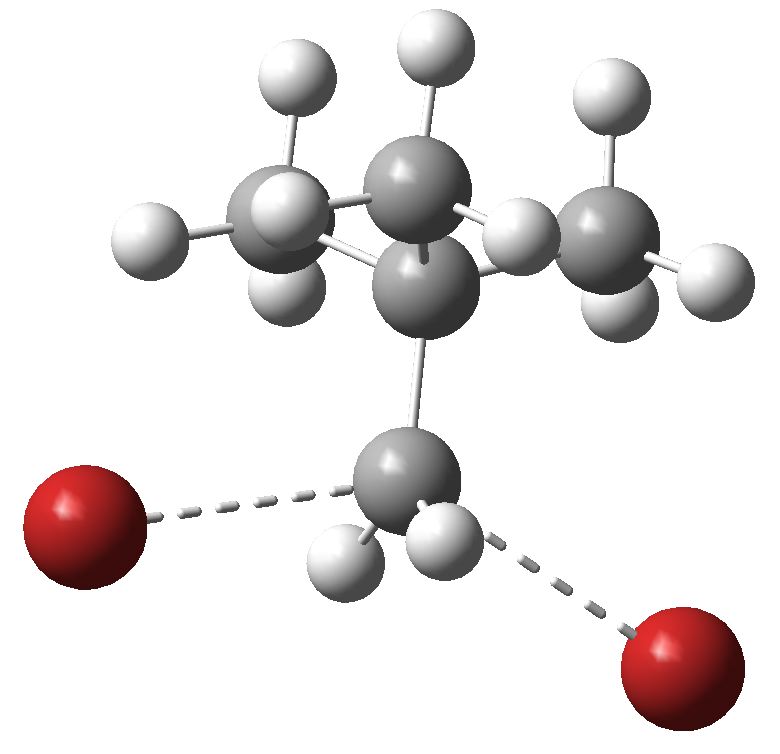
In an earlier post I wrote about the iconic S N 1 solvolysis reaction, and presented a model for the transition state involving 13 water molecules. Here, I follow this up with an improved molecule containing 16 water molecules, and how the barrier for this model compares with experiment.

Every introductory course or text on aromatic electrophilic substitution contains an explanation along the lines of the resonance diagram shown below. With an o / p directing group such as NH 2 , it is argued that negative charge accumulates in those positions as a result of the resonance structures shown. MEP for PhNH2. Click for 3D.The opposite occurs for electron withdrawing groups.