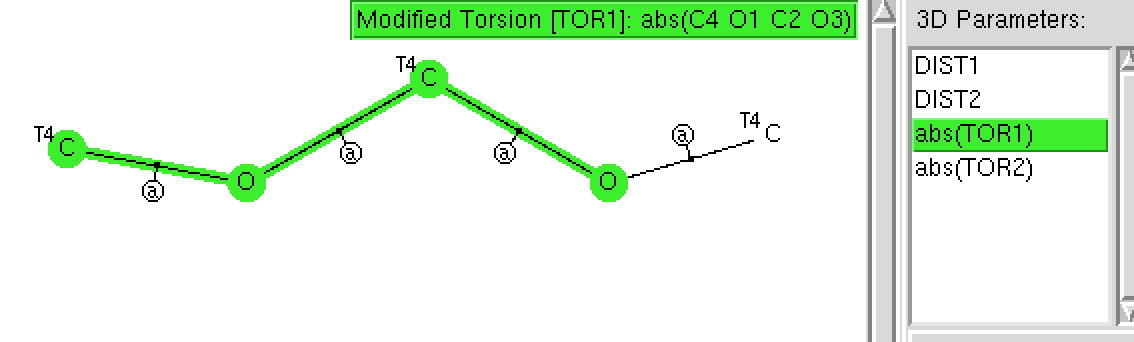
The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs. Here I explore whether crystal structures reflect this effect.