Pubblicato in Henry Rzepa's Blog
Autore Henry Rzepa
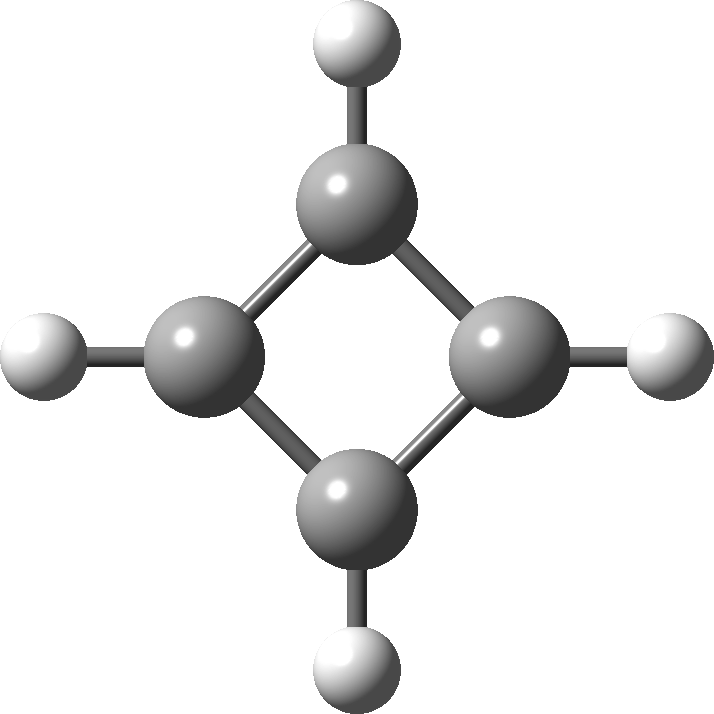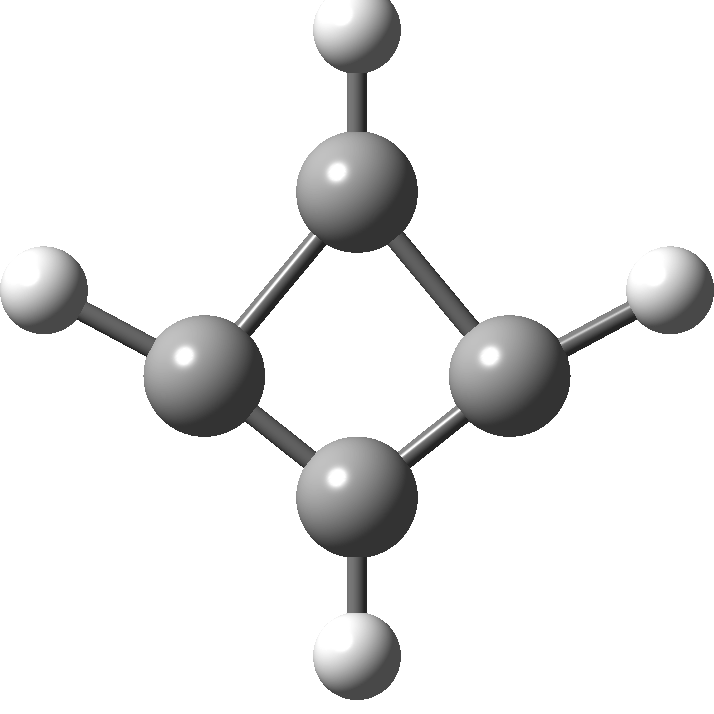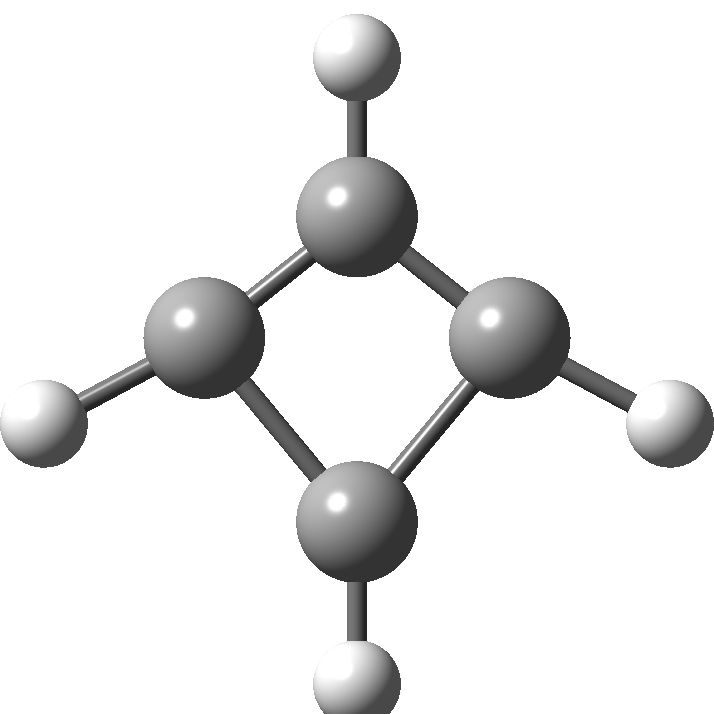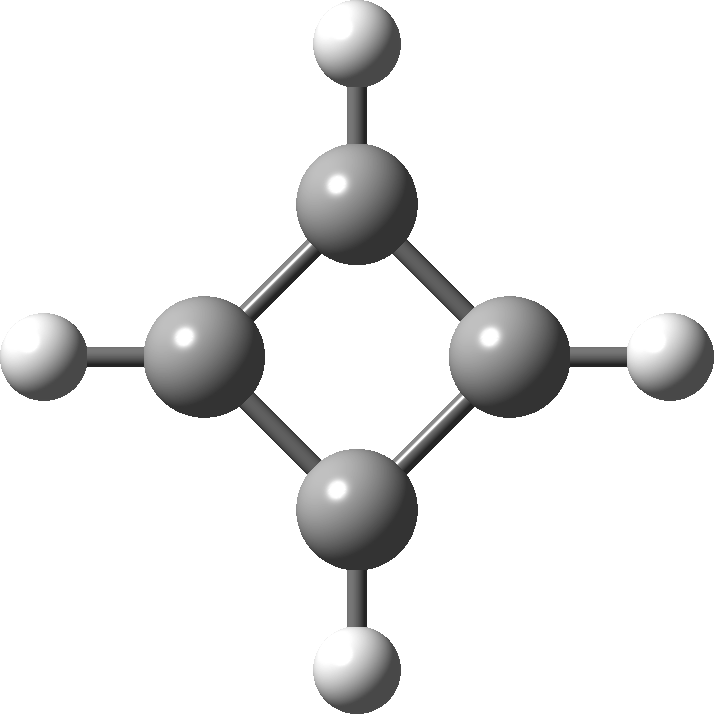
In 2001, Shaik and co-workers published the first of several famous review articles on the topic A Different Story of π-Delocalization.

In 2001, Shaik and co-workers published the first of several famous review articles on the topic A Different Story of π-Delocalization.
It has been found that for diatomic molecules the relation between the bond force constant, k0, and the internuclear distance, re, is quite accurately given by the expression k0(re—dij)3 = 1.86×105, where dij is a constant depending only on the rows in the periodic table in which the two elements comprising the molecule are located. The expression holds not only for the normal state but for all excited states with a few possible exceptions. Some uses of the relation are discussed and an extension to polyatomic molecules is suggested.