Pubblicato in Henry Rzepa's Blog
Autore Henry Rzepa
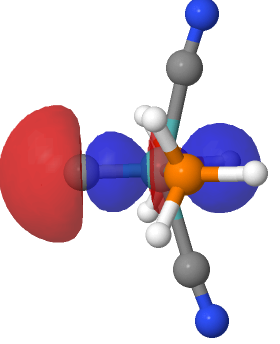
Introductory chemistry will tell us that a triple bond between say two carbon atoms comprises just one bond of σ-axial symmetry and two of π-symmetry. Increasingly mentioned nowadays is the possibility of a quadruple bond between carbon and either itself or a transition metal, as discussed in the previous post. Such a bond comprises TWO bonds of σ-axial symmetry.