The notorious neurotoxin Tetrodotoxin is often chemically represented as a zwitterion, shown below as 1 . This idea seems to originate from a famous article written in 1964 by the legendary organic chemist, Robert Burns Woodward.[cite]10.1351/pac196409010049[/cite] This structure has propagated on to Wikipedia and is found in many other sources.
Messaggi di Rogue Scholar
Increasingly, individual small molecules are having their structures imaged using STM, including cyclo[18]carbon that I recently discussed. The latest one receiving such treatment is Kekulene.[cite]10.1021/jacs.9b07926[/cite] As with cyclo[18]carbon, the point of interest was which of the two resonance structures shown below most closely resembled the measured structure.
If, as a synthetic chemist, you want to invert the configuration of an alcohol in which the OH group is at a chiral centre, then the Mitsunobu reaction has been a stalwart for many years. Now a catalytic version has been published, [cite]10.1126/science.aax3353[/cite] along with a proposed mechanism.
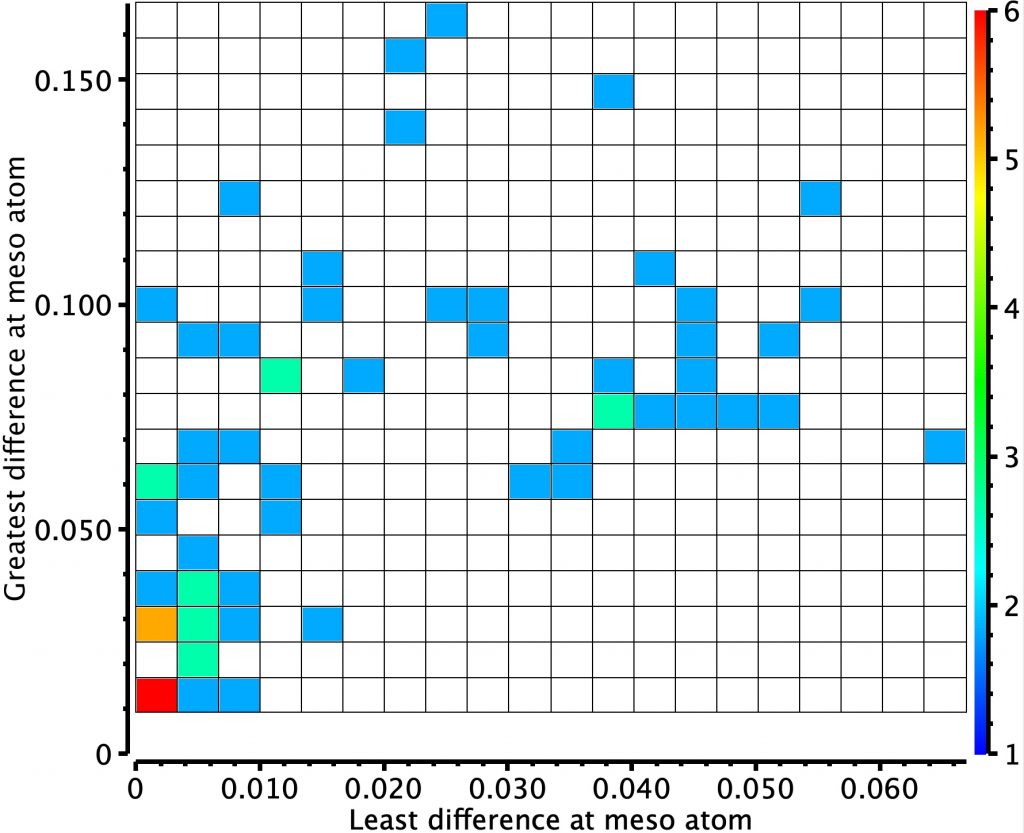
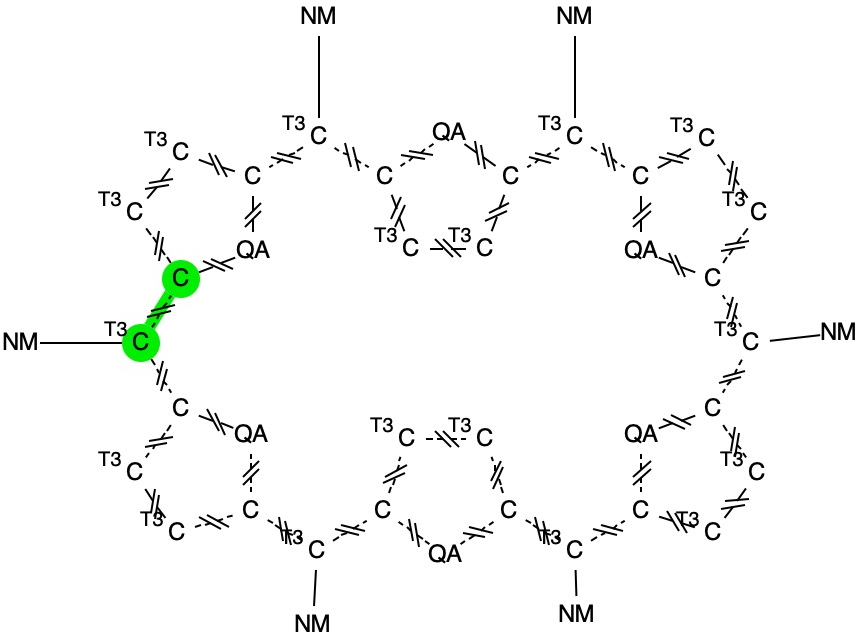
In the previous post, I looked at a class of molecule known as hexaphyrins, inspecting bond length alternation (BLA) at the so-called meso position, the carbon atom joining two pyrrole rings. A search of the difference in bond lengths at this position had shown two significant clusters of crystal structures. Molecules in the bottom left of this diagram shows little or no bond length alternation.
The theme of the last three posts derives from the recently reported claimed experimental observation of bond length alternation (BLA) in cyclo[18]carbon, a ring of just 18 carbon atoms.[cite]10.1126/science.aay1914[/cite] Having found that different forms of quantum calculation seem to find this property particularly difficult to agree upon, not only for cyclocarbon but for twisted lemniscular annulenes (which contain CH rather than just C
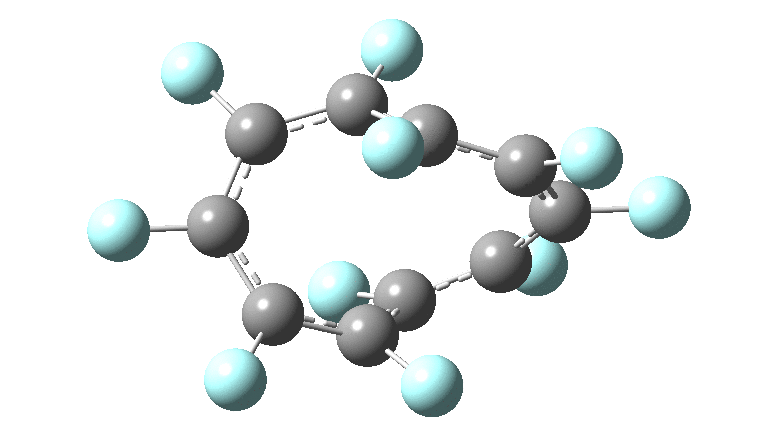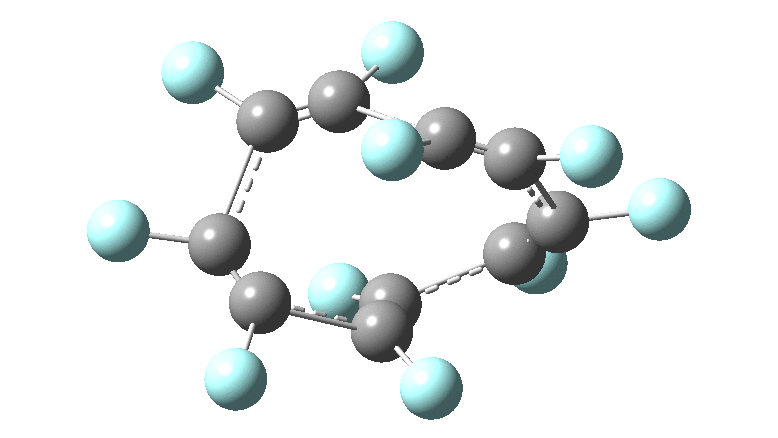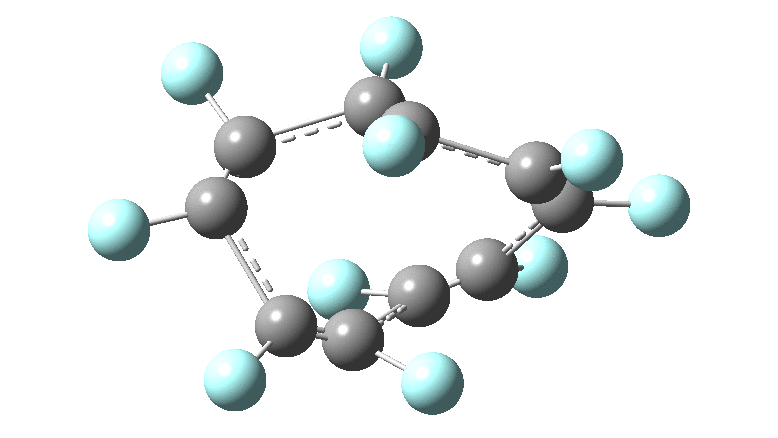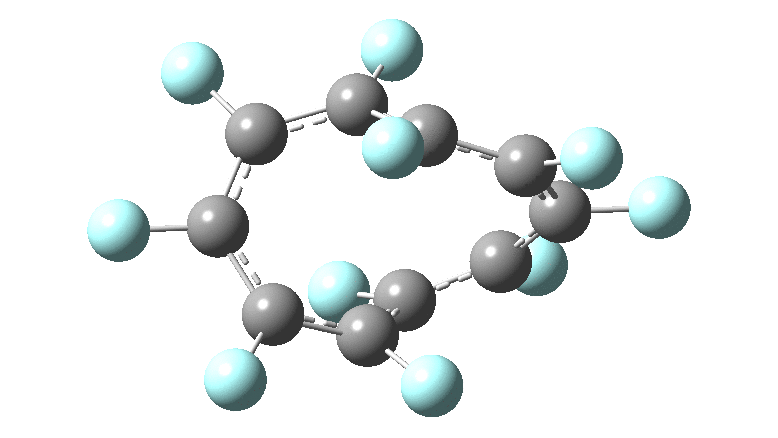
In the previous posts, I tried to track down the onset of bond length alternation ( BLA ) as a function of ring size in aromatic cyclocarbons, finding the answer varied dramatically depending on the type of method used to calculate it. So here I change the system to an unusual kind of aromatic ring, the leminiscular or figure-eight annulene series. ♥ I explore the Kekulé vibration for such species
In the previous post, I looked at the so-called Kekulé vibration of cyclo[18]carbon using various quantum methods and basis sets. Because some of these procedures can take a very long time, I could not compare them using the same high-quality consistent atom basis set for the carbon (Def2-TZVPP). Here I try to start to do this using the smaller six and ten carbon rings to see what trends might emerge.
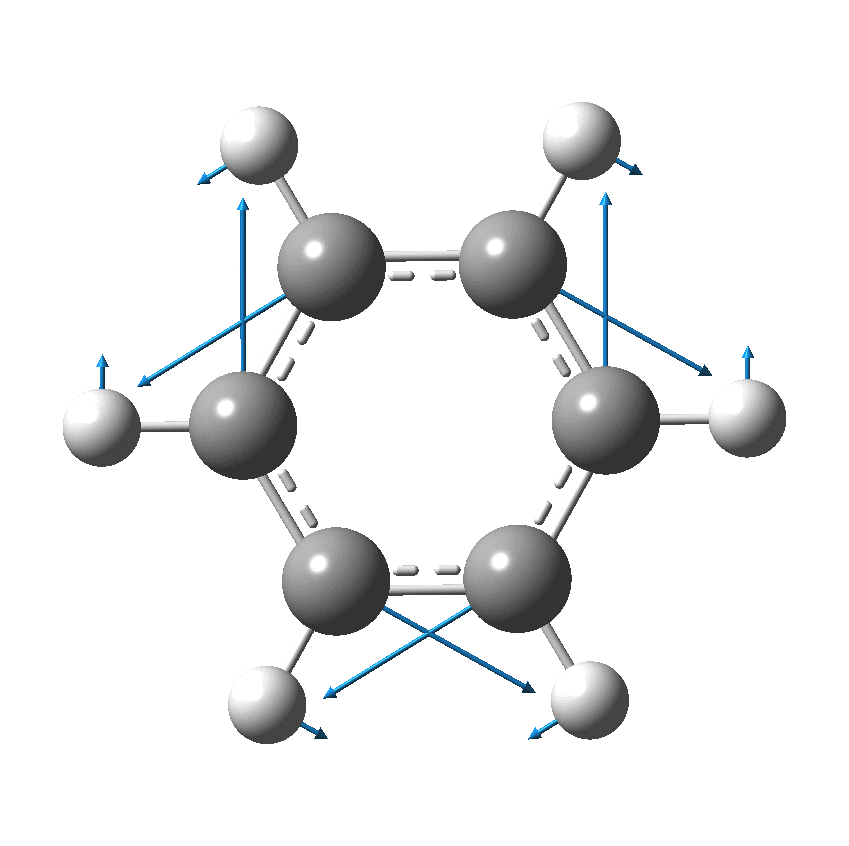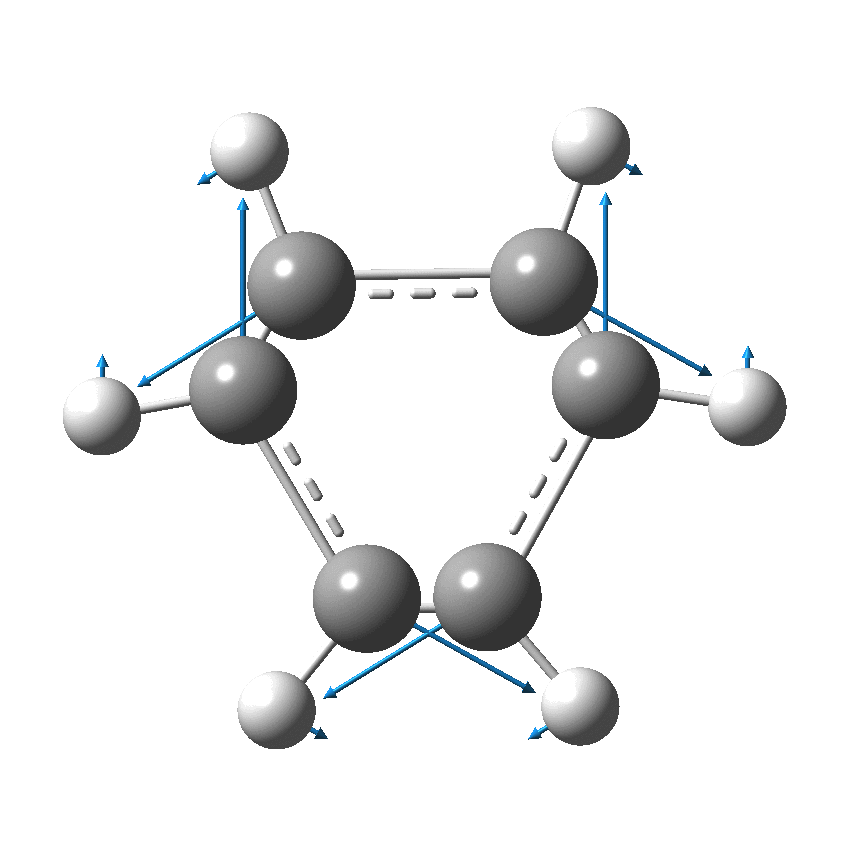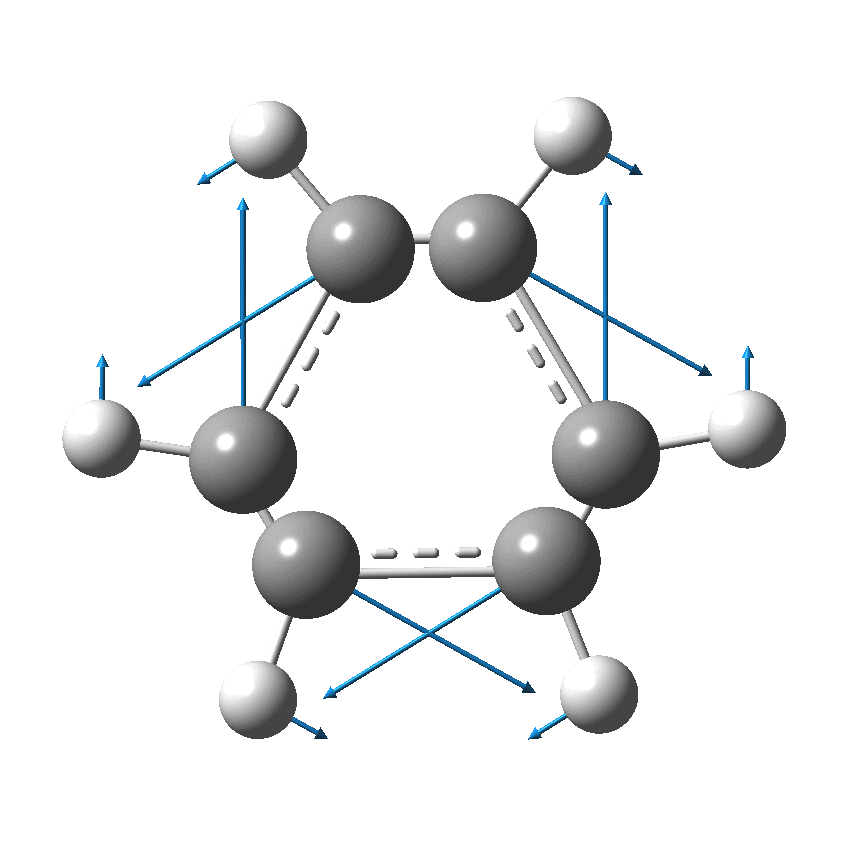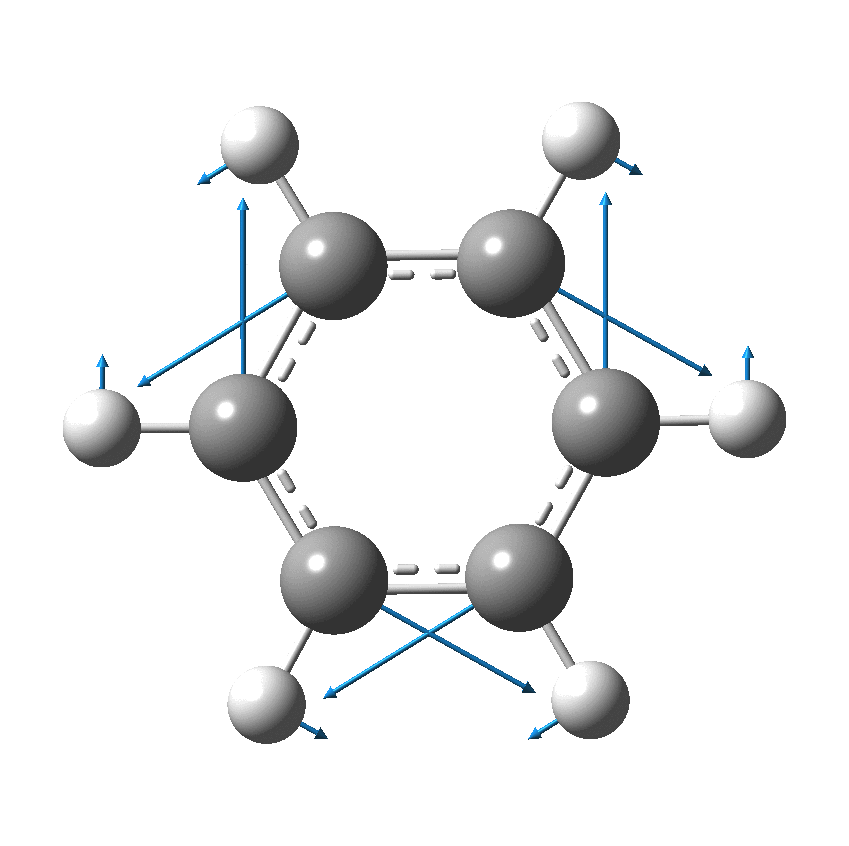
I have discussed the vibration in benzene known as the Kekulé mode in other posts, the first of which was all of ten years ago.
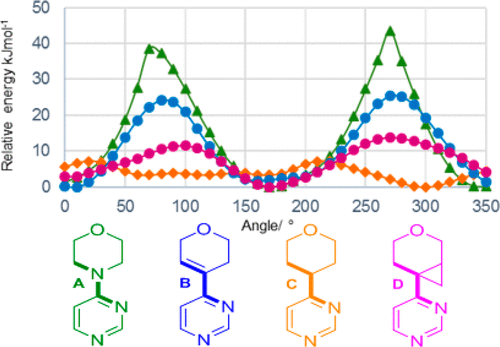
In the pipeline reports on an intriguing new ring system acting as an isostere for morpholine. I was interested in how the conformation of this ring system might be rationalised electronically and so I delved into the article.[cite]10.1021/acs.jmedchem.9b00348[/cite] Here I recount what I found.
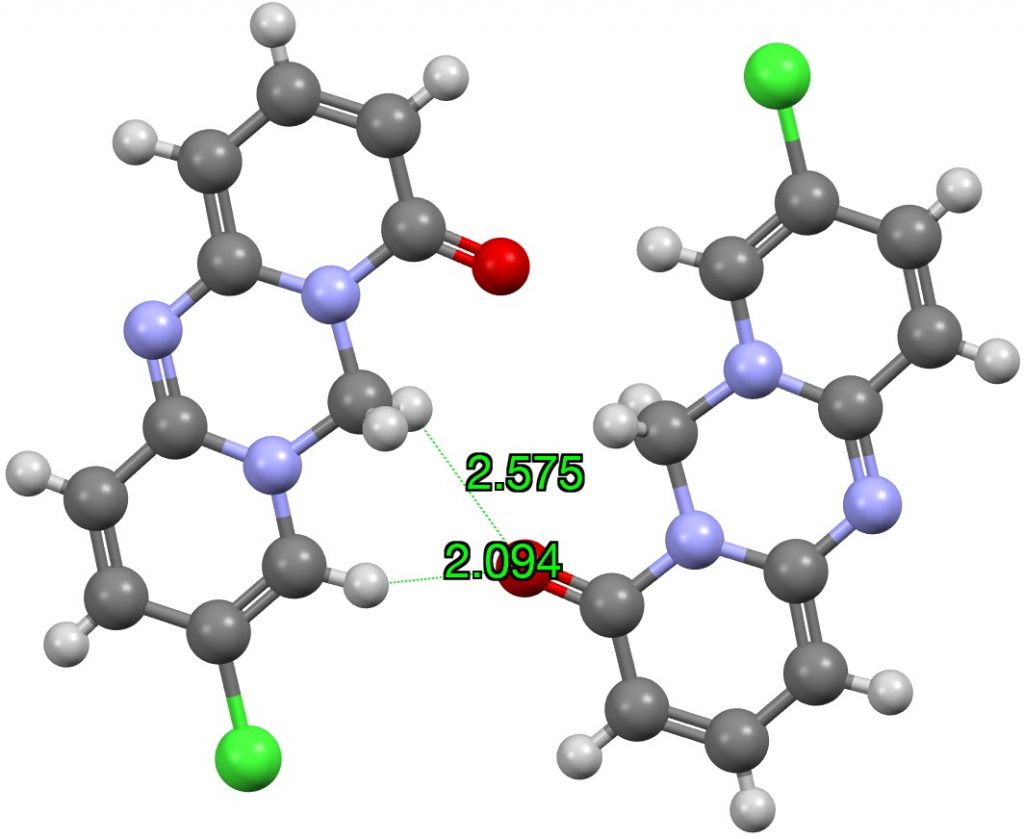
I have previously looked at the topic of hydrogen bonding interactions from the hydrogen of chloroform Here I generalize C-H…O interactions by conducting searches of the CSD (Cambridge structure database) as a function of the carbon hybridisation. I am going to jump straight to a specific molecule XEVJIR (DOI: 10.5517/cc5fgpq) identified from the searches appended to this post as interesting for further