I was about to call this blog post From spreadsheets to RDF , after the post last week. But then I decided to just use the pattern I typically use. Why I wanted to use that shorter term in the first place was that one of the thing I like about the AMBIT software (of OpenTox and eNanoMapper fame) is its RDF support (see doi:10.1186/1756-0500-4-487). But RDF, ontologies, those are hard things.
Messaggi di Rogue Scholar
In the previous post, I explored the so-called “impossible” molecule methanetriol. It is regarded as such because the equilbrium resulting in loss of water is very facile, being exoenergic by ~14 kcal/mol in free energy. Here I explore whether changing the substituent R could result in suppressing the loss of water and stabilising the triol.

Making something FAIR is hard, particularly when you do more than making something findable. We’ve seen before that making something usefully findable requires deep indexing, and already that continues to be difficult, because we are not seeing it enough. So, when I thought convert a paper led by Hoet’s lab in Leuven into machine-actionable RDF to make it FAIR, I gravely underestimated the amount of work.
Publishing grant proposal is still not very common. The proposal published in Research Ideas and Outcomes) (doi:10.3897/rio.10.e124884) for the NWO Open Science grant for the CDK is, however, not the first and hopefully not the last. Interestingly, it is already cited in (the German) Wikipedia. It is used there to support a statement which tools use the Chemistry Development Kit.
What constitutes an “impossible molecule”? Well, here are two, the first being the topic of a recent article[cite]10.1021/jacs.4c02637[/cite]. The second is a favourite of organic chemistry tutors, to see if their students recognise it as an unusual (= impossible) form of a much better known molecule. Perhaps we could define impossible molecules into two slightly different classes.
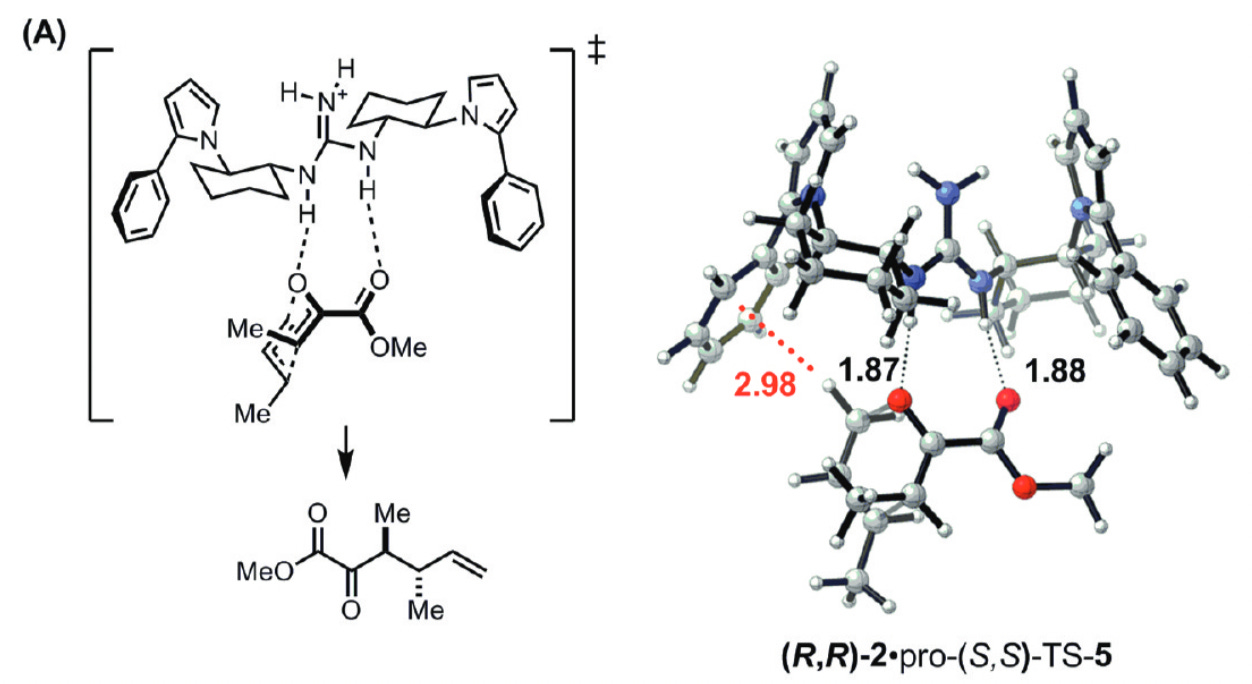
Much molecular design today can be boiled down to “put the right functional groups in exactly the right places.” In catalysis, proper positioning of functional groups to complement developing charge or engage in other stabilizing non-covalent interactions with the transition state can lead to vast rate accelerations.
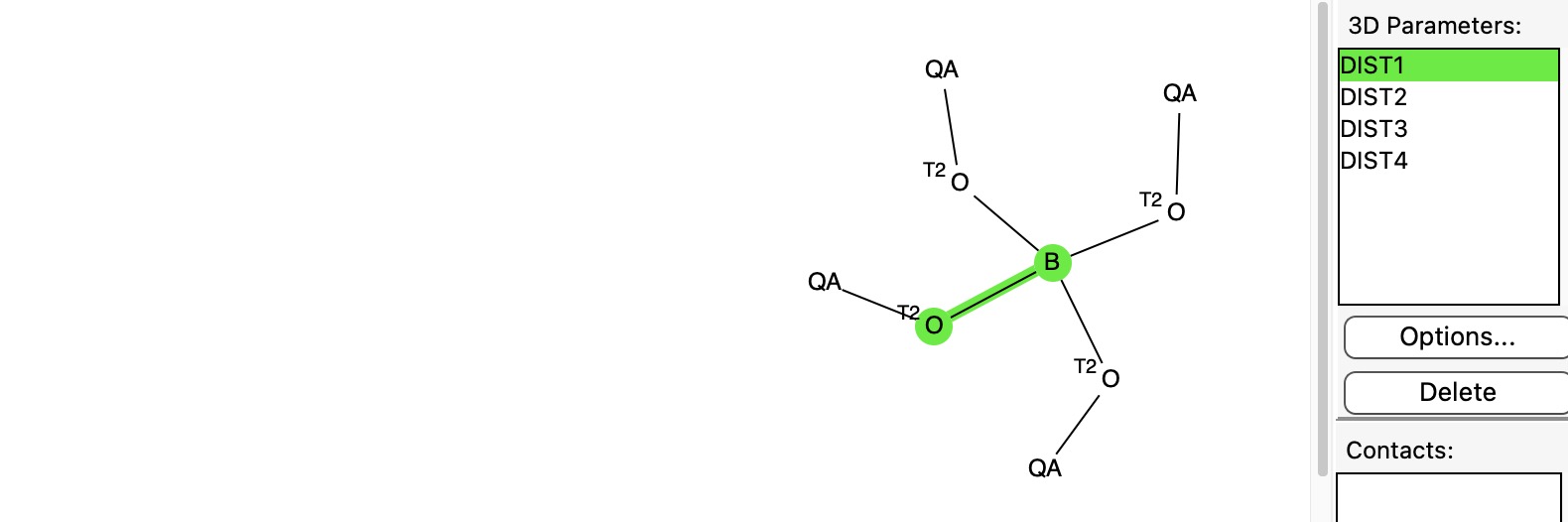
In an earlier post, I discussed[cite]10.59350/dfkt5-k2b20[/cite] a phenomenon known as the “anomeric effect” exhibited by tetrahedral carbon compounds with four C-O bonds. Each oxygen itself bears two bonds and has two lone pairs, and either of these can align with one of three other C-O bonds to generate an anomeric effect. Here I change the central carbon to a boron to explore what happens, as indeed I promised earlier.
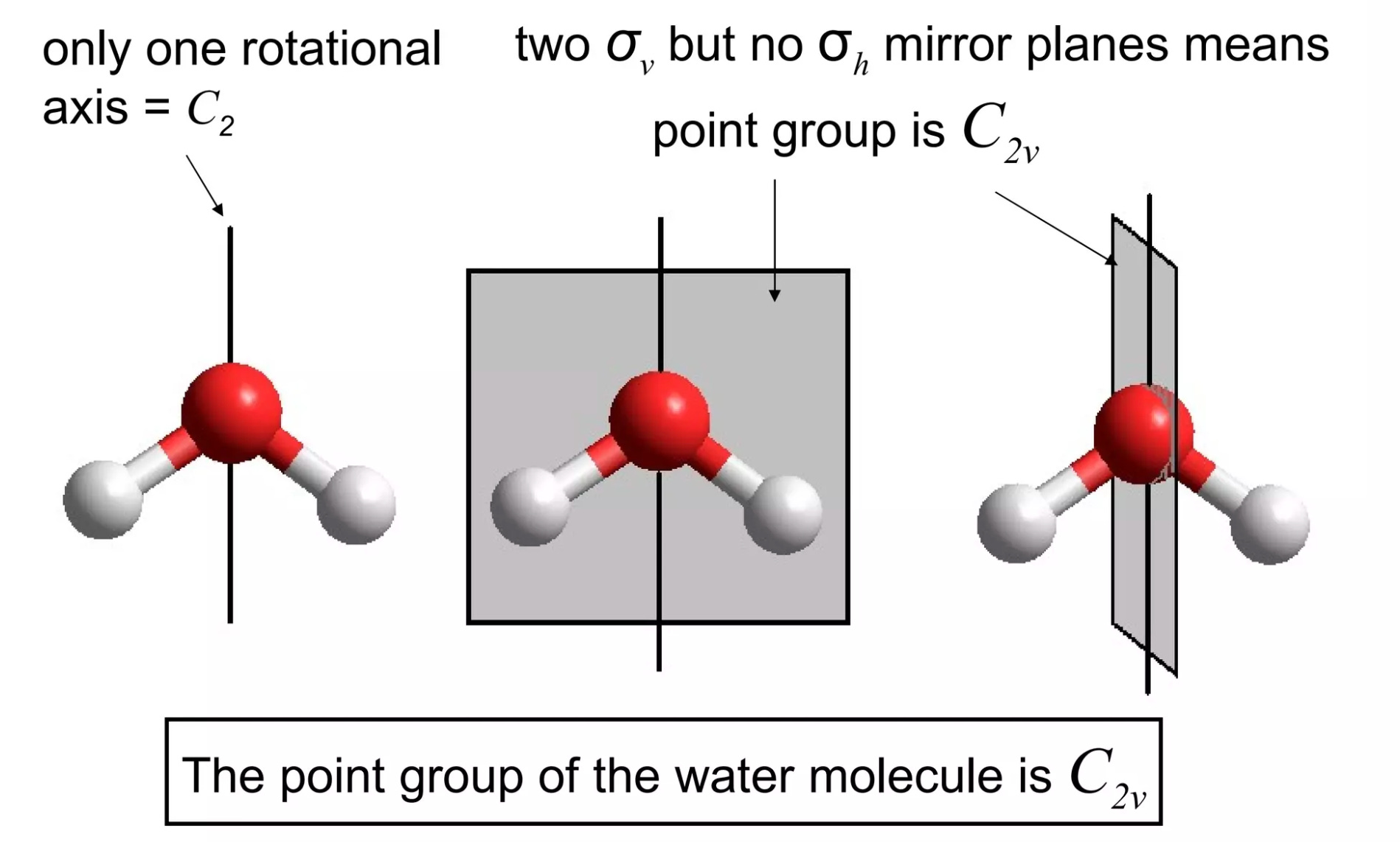
Pure mathematics has all sorts of unexpected connections to other fields, and chemistry is no exception. One example of this is group theory: while I never delved deeply enough into math to actually study group theory as its own field, I've had to learn how to assign point groups to three-dimensional objects for several inorganic chemistry classes.
We recently got awarded our second NWO Open Science grant (OSF23.2.097), this time for the Chemistry Development Kit (CDK). “We” here is me and Alyanne de Haan, René van der Ploeg, and Marc Teunis from Hogeschool Utrecht. The proposal has been submitted for public dissemination in RIO Journal, like we did with the first NWO Open Science grant.
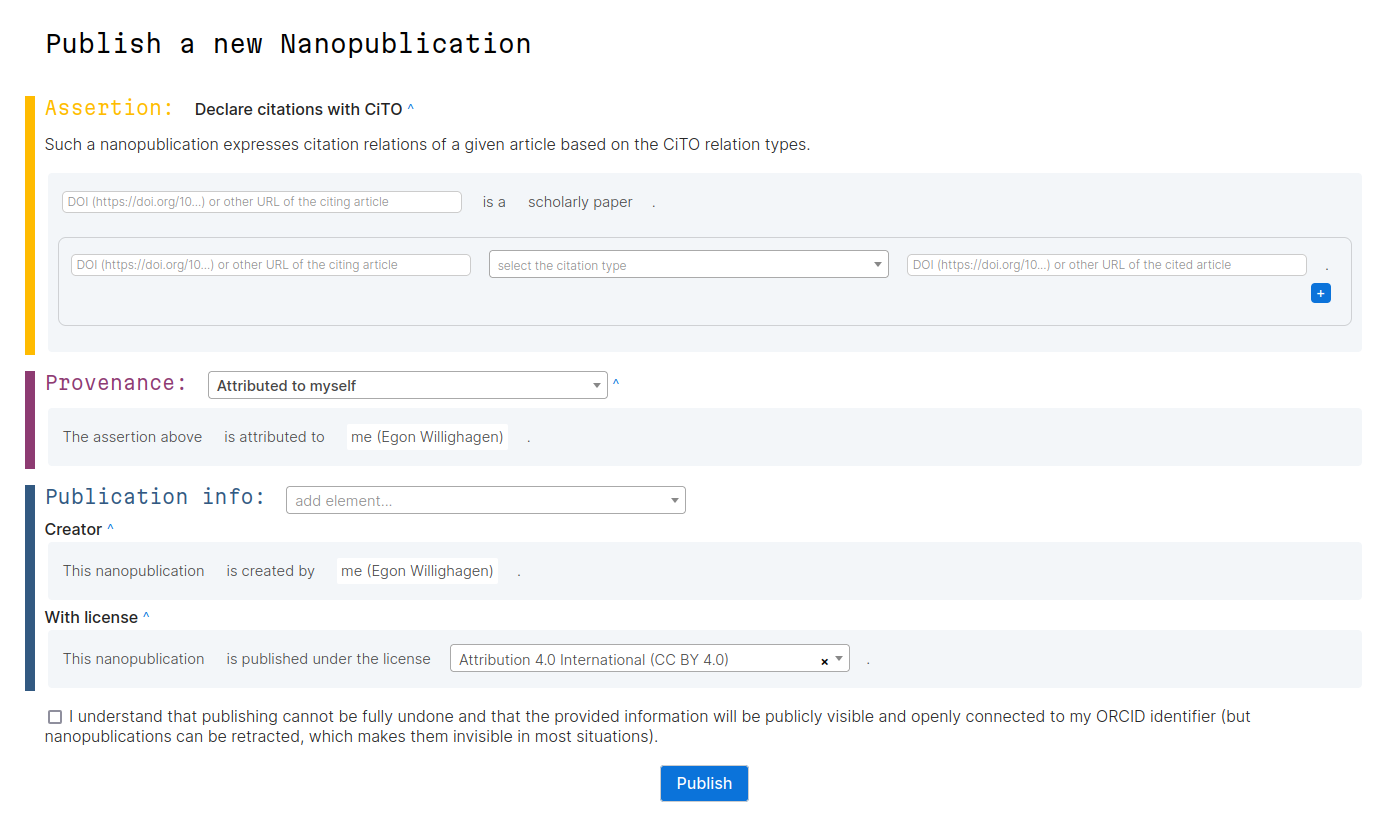
During the Open Science Retreat I organized a short session where we looking into typing citation intentions using a new nanopublication template. First, let’s describe nanopublications (originally used in doi:10.3233/ISU-2010-0613) a bit. Scholia gives a nice overview of (macro?)publications on the topic.