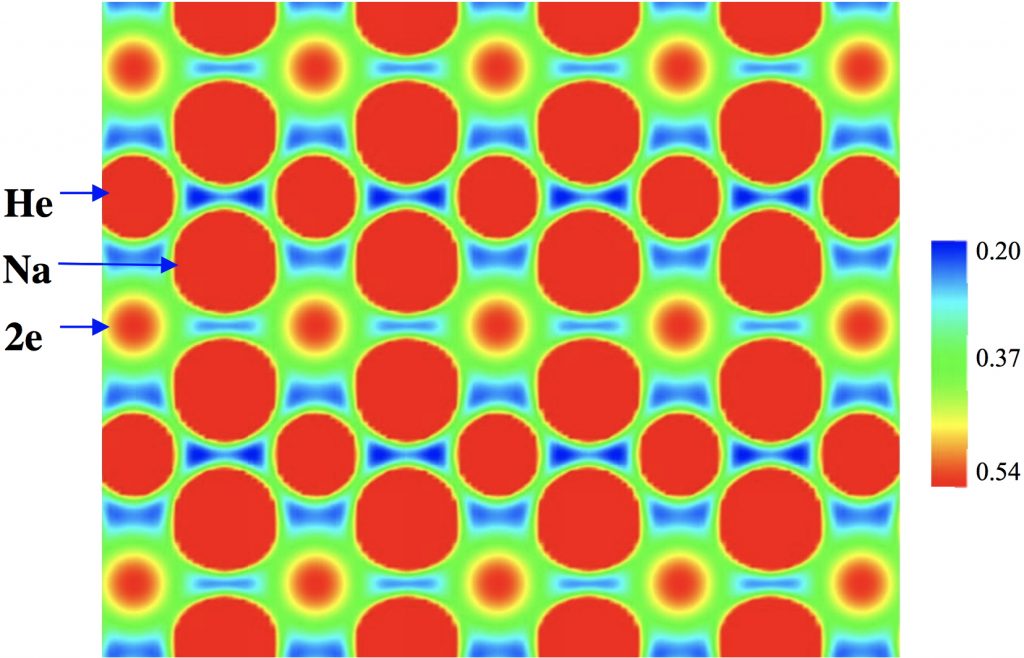
On February 6th I was alerted to this intriguing article[cite]10.1038/nchem.2716[/cite] by a phone call, made 55 minutes before the article embargo was due to be released. Gizmodo wanted to know if I could provide an (almost) † instant ‡ quote. After a few days, this report of a stable compound of helium and sodium still seems impressive to me and I now impart a few more thoughts here.