I am completing my survey of the vote for molecule of the year candidates, which this year seems focused on chemical records of one type or another. The first article[cite]10.1002/chem.201601916[/cite] reports striving towards creating a molecule covering a complete column of the period table.
Messaggi di Rogue Scholar
This, the fourth candidate provided by C&EN for a vote for the molecule of the year as discussed here, lays claim to the World’s most polar neutral molecule (system 1 shown below).[cite]10.1002/anie.201508249[/cite] Here I explore a strategy for extending that record.
Here is a third candidate for the C&EN “molecule of the year” vote. This one was shortlisted because it is the first example of a metal-nitrogen complex exhibiting single, double and triple bonds from different nitrogens to the same metal[cite]10.1039/c5sc04608d[/cite] (XUZLUB has a 3D display available at DOI: 10.5517/CC1JYY6M). Since no calculation of its molecular properties was reported, I annotate some here.
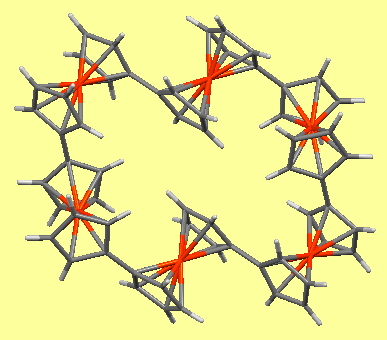
Chemical and engineering news (C&EN) is asking people to vote for their molecule of the year from six highlighted candidates. This reminded me of the history of internet-based “ molecules of the moment ”. It is thought that the concept originated in December 1995 here at Imperial and in January 1996 at Bristol University by Paul May and we were joined by Karl Harrison at Oxford shortly thereafter.
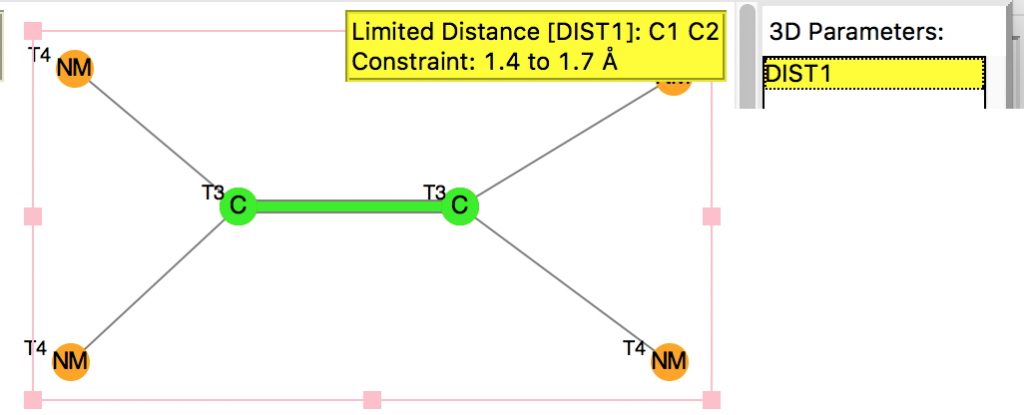
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds. The query restricts the search to each carbon having just two non-metallic substituents. To avoid conjugation with these, they each are 4-coordinated; the carbons themselves are three-coordinated.
In an earlier post, I searched for small C-C-C angles, finding one example that was also accompanied by an apparently exceptionally long C-C bond (2.18Å). But this arose from highly unusual bonding giving rise not to a single bond order but one closer to one half! How long can a “normal” (i.e single) C-C bond get, a question which has long fascinated chemists. A naive search of the CSD is not as straightforward as it seems.

Another conference, a Cambridge satellite meeting of OpenCon, and I quote here its mission: “OpenCon is a platform for the next generation to learn about Open Access, Open Education, and Open Data, develop critical skills, and catalyze action toward a more open system of research and education” targeted at students and early career academic professionals. But they do allow a few “late career” professionals to attend as well!
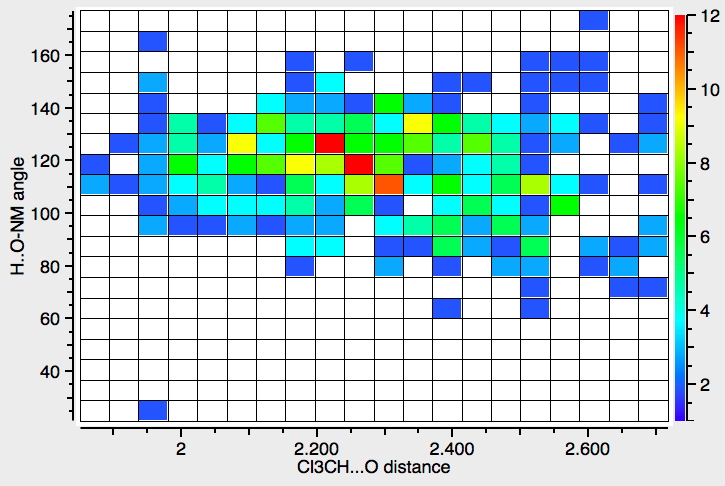
Chloroform, often in the deuterated form CDCl 3 , is a very common solvent for NMR and other types of spectroscopy. Quantum mechanics is increasingly used to calculate such spectra to aid assignment and the solvent is here normally simulated as a continuum rather than by explicit inclusion of one or more chloroform molecules. But what are the features of the hydrogen bonds that form from chloroform to other acceptors?
This is sent from the Pidapalooza event in Reykjavik, Iceland, and is a short collection of notable things I learnt or which attracted my attention. Firstly, what IS PIDapalooza [cite]10.5438/11.0001[/cite]? Well, it’s all about persistent identifiers, but don’t let that put you off! Another way of putting it is that it’s a way of finding things scientific on the Web.
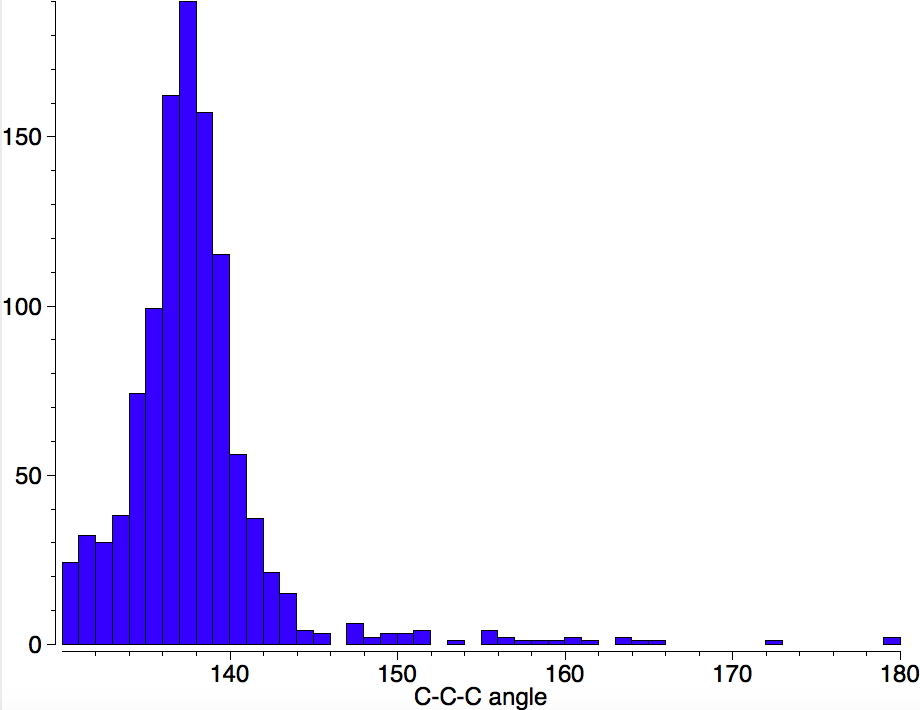
I am now inverting the previous question by asking what is the largest angle subtended at a chain of three connected 4-coordinate carbon atoms ? Let’s see if further interesting chemistry can be unearthed. Specifying only angles > 130°, the following distribution is obtained.