A scalemic molecule is the term used by Eliel to describe any non-racemic chiral compound.
Messaggi di Rogue Scholar
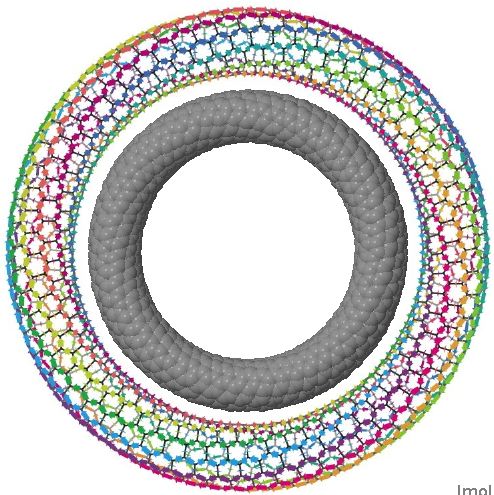
The interface between physics, chemistry (and materials science) can be a fascinating one. Here I show a carbon nanotorus, devised by physicists[cite]10.1103/PhysRevLett.88.217206[/cite] a few years ago. It is a theoretical species, and was predicted to have a colossal paramagnetic moment . Carbon nanotorus.
Understanding why and how proteins fold continues to be a grand challenge in science. I have described how Wrinch in 1936 made a bold proposal for the mechanism, which however flew in the face of much of then known chemistry.

Science is about making connections. Plenty are on show in Watson and Crick’s famous 1953 article on the structure of DNA[cite]10.1038/171737a0[/cite] but often with the tersest of explanations. Take for example their statement “ Both chains follow right-handed helices ”. Where did that come from?
Our understanding of science mostly advances in small incremental and nuanced steps (which can nevertheless be controversial) but sometimes the steps can be much larger jumps into the unknown, and hence potentially more controversial as well. More accurately, it might be e.g. relatively unexplored territory for say a chemist, but more familiar stomping ground for say a physicist.

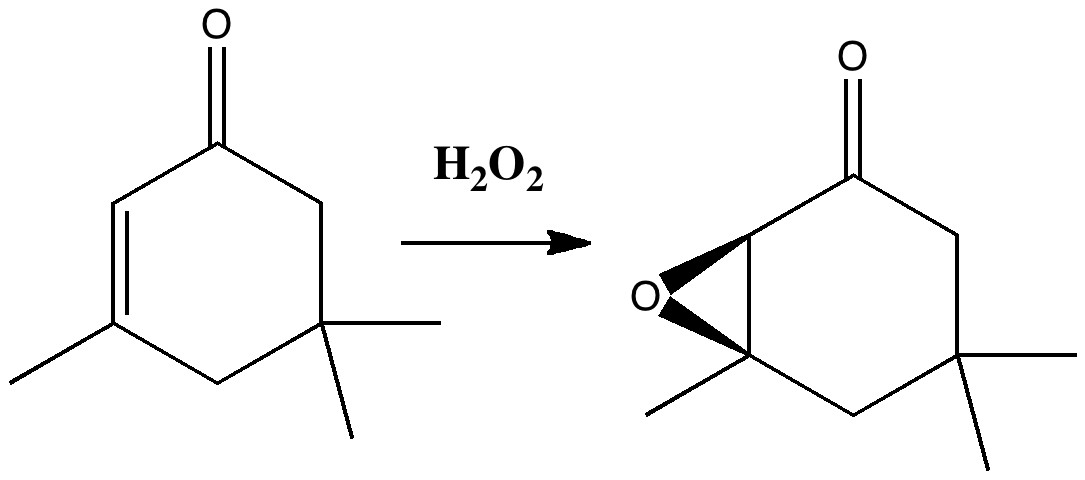
Stereo-induction is, lets face it, a subtle phenomenon. The ratio of two stereoisomers formed in a reaction can be detected very accurately by experiment, and when converted to a free energy difference using ΔG = -RT Ln K, this can amount to quite a small value (between 0.5 – 1.5 kcal/mol). Can modelling reproduce effects originating from such small energy differences?
A conjugated, (apparently) aromatic molecular trefoil might be expected to have some unusual, if not extreme properties. Here some of these are explored. The first is the vibrational spectrum. With 144 atoms for this molecule, it has 426 vibrational modes, but one is highlighted below. This is the mode that moves the atoms in accord with the Kekulé resonance. If real, this mode resists such alternation.

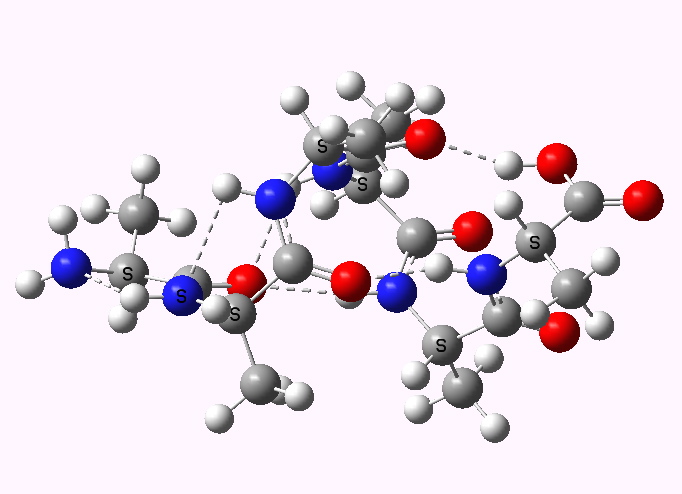
In the first part of the post on this topic, I described how an asymmetric sulfoxide could be prepared as a pure enantiomer using a chiral oxygen transfer reagent. In the second part, we now need to deliver a different group, cyano, to a specific face of the previously prepared sulfoxide-imine.

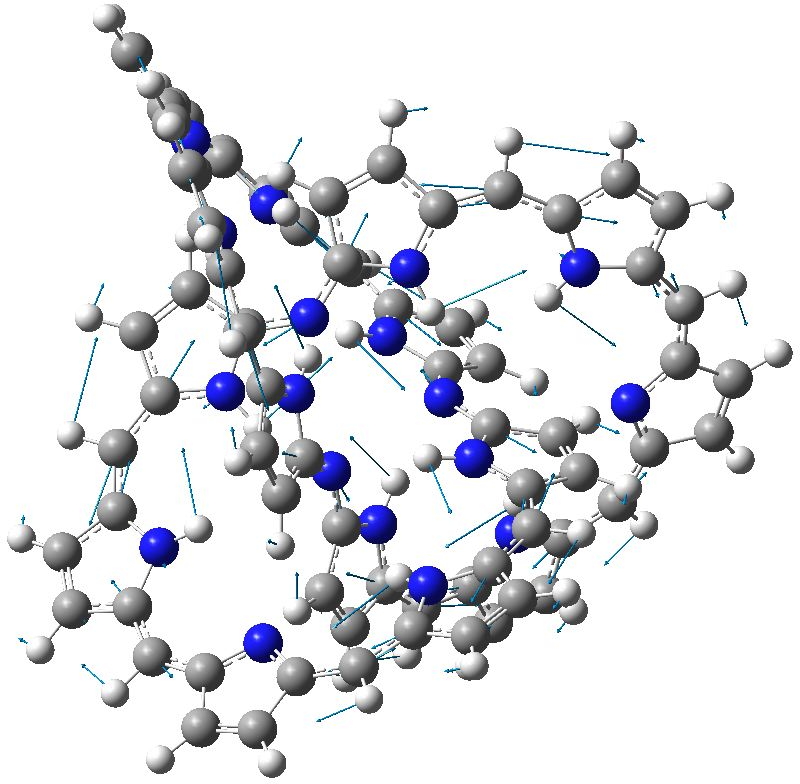
In the previous post, it was noted that Möbius annulenes are intrinsically chiral, and should therefore in principle be capable of resolution into enantiomers. The synthesis of such an annulene by Herges and co-workers was a racemic one; no attempt was reported at any resolution into such enantiomers. Here theory can help, since calculating the optical rotation [α]D is nowadays a relatively reliable process for rigid molecules.

Much like climbing Mt. Everest because its there, some hypothetical molecules are just too tantalizing for chemists to resist attempting a synthesis. Thus in 1964, Edgar Heilbronner speculated on whether a conjugated annulene ring might be twistable into a Möbius strip. It was essentially a fun thing to try to do, rather than the effort being based on some anticipated (and useful) property it might have.