The autoionization of water involves two molecules transfering a proton to give hydronium hydroxide, a process for which the free energy of reaction is well known. Here I ask what might happen with the next element along in the periodic table, F. I have been unable to find much about the autoionization of HF in the literature;
Messaggi di Rogue Scholar
Earlier, I constructed a possible model of hydronium hydroxide, or H3O+.OH– One way of assessing the quality of the model is to calculate the free energy difference between it and two normal water molecules and compare the result to the measured difference. Here I apply a further test of the model using isotopes.
In the previous post, I pondered how a substituent (X below) might act to slow down the hydrolysis of an acetal. Here I extend that by probing the role of water molecules in the mechanism of acetal hydrolysis.
In London, one has the pleasures of attending occasional one day meetings at the Burlington House, home of the Royal Society of Chemistry. On November 5th this year, there was an excellent meeting on the topic of *Challenges in Catalysis, *and you can see the speakers and (some of) their slides here.
I promised that the follow-up to on the topic of Birch reduction would focus on the proton transfer reaction between the radical anion of anisole and a proton source, as part of analysing whether the mechanistic pathway proceeds O or M . To add some context, Hammond’s postulate [cite]10.1021/ja01607a027[/cite] states that “ the structure of a transition state resembles that of the species nearest
The Birch reduction is a classic method for partially reducing e.g. aryl ethers using electrons (from sodium dissolved in ammonia) as the reductant rather than e.g. dihydrogen. As happens occasionally in chemistry, a long debate broke out over the two alternative mechanisms labelled O (for ortho protonation of the initial radical anion intermediate) or M (for meta protonation).
Singleton and co-workers have produced some wonderful work showing how dynamic effects and not just transition states can control the outcome of reactions. Steve Bachrach’s blog contains many examples, including this recent one. This shows that tolyl thiolate (X=Na) reacts with the dichlorobutenone to give two substitution products in a 81:19 ratio.
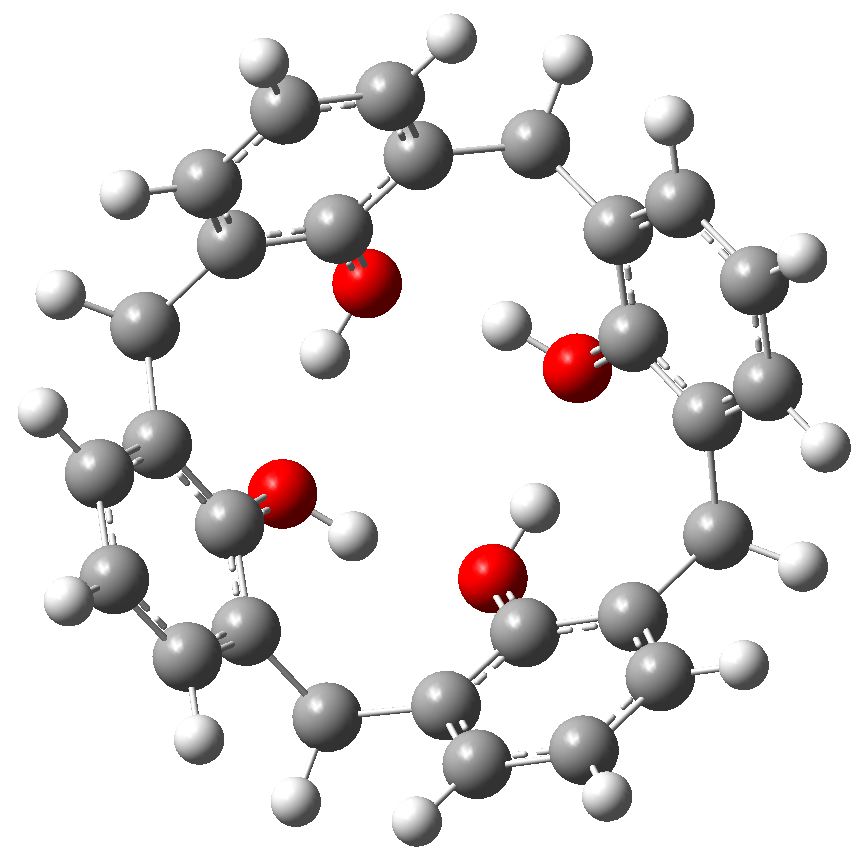
This story starts with a calixarene, a molecule (suitably adorned with substituents) frequently used as a host to entrap a guest and perchance make the guest do something interesting. Such a calixarene was at the heart of a recent story where an attempt was made to induce it to capture cyclobutadiene in its cavity.