The best known example of the gauche effect is 1,2-difluoroethane, which exhibits a relatively small preference of ~0.5 kcal/mol for this conformer over the anti orientation, which is also a minimum. But FSSF, which I discussed in the previous post, beats this hands down!
Messaggi di Rogue Scholar
The equilibrium for the hydration of a ketone to form a gem-diol hydrate is known to be highly sensitive to substituents. Consider the two equilibria: For propanone, it lies almost entirely on the left, whereas for the hexafluoro derivative it is almost entirely on the right. The standard answer to this is that electron-withdrawing substituents destabilize the carbonyl compound more than the hydrate.
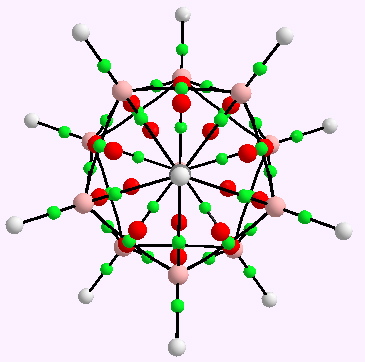
A little while ago, I speculated (blogs are good for that sort of thing) about hexavalent carbon, and noted how one often needs to make (retrospectively) obvious connections between two different areas of chemistry. That post has attracted a number of comments in the two years its been up, along the lines: what about carboranes? So here I have decided to explore that portal into boron chemistry.
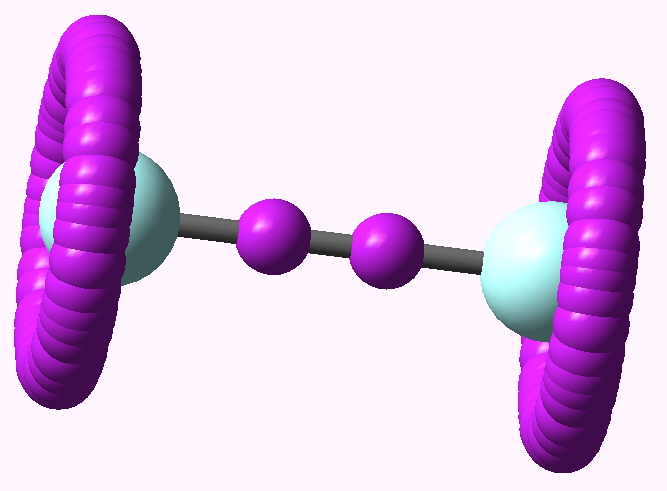
In the previous post, I ruminated about how chemists set themselves targets. Thus, having settled on describing regions between two (and sometimes three) atoms as bonds , they added a property of that bond called its order . The race was then on to find molecules which exhibit the highest order between any particular pair of atoms.