This is another of those textbook reactions, involving reaction of a carbonyl compound with a phosphonium ylid to form an alkene and a phosphine oxide. The reaction continues to be frequently used, in part because it can be highly stereospecific. Thus the standard version tends to give Z -alkenes with good specificity, and is thought to proceed via an oxaphosphatane 4-ring intermediate.
Messaggi di Rogue Scholar
The Curtius reaction is represented in most chemistry texts and notes as following path (a) below. It is one of a general class of thermally induced rearrangement which might be described as elimination/migration (in a sense similar to this ring contraction migration/elimination), in this case implicating a nitrene intermediate if the two steps occur consecutively.

Two years ago, I discussed how curly arrow pushing is taught, presenting four different ways of showing the arrows. One of the comments posted to that blog suggested that all of the schemes shown below were deficient in one aspect. Curly arrow pushing The issues were the stereo and regiochemistry. In particular, the diagram above carries no explicit information about the symmetry of the electrons from which the first arrow originates;
Little did I imagine, when I discovered the original example of using curly arrows to express mechanism, that the molecule described there might be rather too anarchic to use in my introductory tutorials on organic chemistry. Why? It simply breaks the (it has to be said to some extent informal) rules!
In the preceding post, I described a fascinating experiment and calculation by Bogle and Singleton, in which the trajectory distribution of molecules emerging from a single transition state was used to rationalise the formation of two isomeric products 2 and **3. ** In the present post, I explore possible consequences of including a sodium cation (X=Na + below) in the computational
Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published[cite]10.1039/C39920001323[/cite]. That was then. This is now.
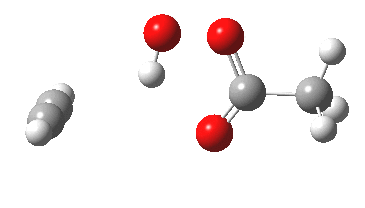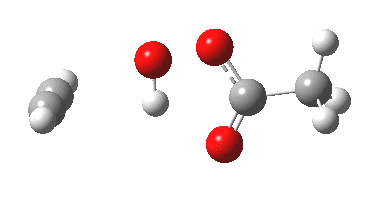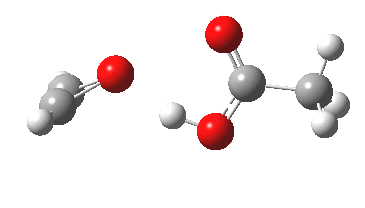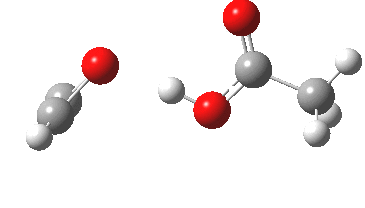
Sometimes, connections between different areas of chemistry just pop out (without the help of semantic web tools, this is called serendipity). So here, I will try to join up some threads which emerge from previous posts. I had noted that antiaromaticity in cyclopropenium anion is lessened by the system adopting gross geometric distortions, which take the anionic lone pair out of conjugation from the ring.
The reaction between a carbene and an alkene to form a cyclopropane is about as simple a reaction as one can get. But I discussed before how simple little molecules (cyclopropenyl anion) can hold surprises. So consider this reaction: Transition state for reaction between ethene and dichlorocarbene.
This is a continuation of the previous post exploring the transition state geometries of various types of ring closure as predicted by Baldwin’s rules. I had dealt with bond formation to a trigonal (sp 2 ) carbon; now I add a digonal (sp) example (see an interesting literature variation).
If you have not previously visited, take a look at Nick Greeves’ ChemTube3D , an ever-expanding gallery of reactions and their mechanisms. The 3D is because all molecules are offered with X, Y and z coordinates. You also get arrow pushing ‡ in 3D. Here, I argue that we should adopt Einstein, and go to the space-time continuum!