The previous posts produced discussion about the dipole moments of highly polar molecules.

The previous posts produced discussion about the dipole moments of highly polar molecules.
A project fork is defined (in computing) as creating a distinct and separate strand from an existing (coding) project. Here I apply the principle to the polar azulene 4 explored in an earlier post, taking m-benzyne as a lower homologue of azulene as my starting point. m-Benzyne is a less stable 1,3 isomer of o-benzyne (1,2-dehydrobenzene), and is often represented as a 1,3-biradical of 1,3-dehydrobenzene.
I am completing my survey of the vote for molecule of the year candidates, which this year seems focused on chemical records of one type or another. The first article[cite]10.1002/chem.201601916[/cite] reports striving towards creating a molecule covering a complete column of the period table.
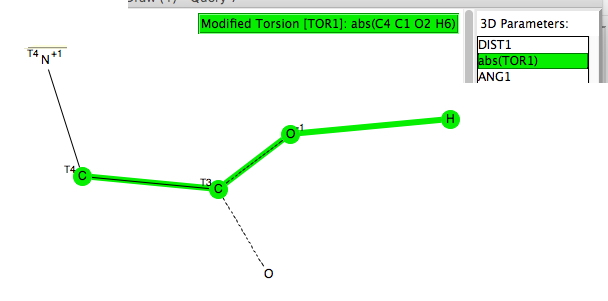
This, the fourth candidate provided by C&EN for a vote for the molecule of the year as discussed here, lays claim to the World’s most polar neutral molecule (system 1 shown below).[cite]10.1002/anie.201508249[/cite] Here I explore a strategy for extending that record.
Here is a third candidate for the C&EN “molecule of the year” vote. This one was shortlisted because it is the first example of a metal-nitrogen complex exhibiting single, double and triple bonds from different nitrogens to the same metal[cite]10.1039/c5sc04608d[/cite] (XUZLUB has a 3D display available at DOI: 10.5517/CC1JYY6M). Since no calculation of its molecular properties was reported, I annotate some here.
Chemical and engineering news (C&EN) is asking people to vote for their molecule of the year from six highlighted candidates. This reminded me of the history of internet-based “ molecules of the moment ”. It is thought that the concept originated in December 1995 here at Imperial and in January 1996 at Bristol University by Paul May and we were joined by Karl Harrison at Oxford shortly thereafter.
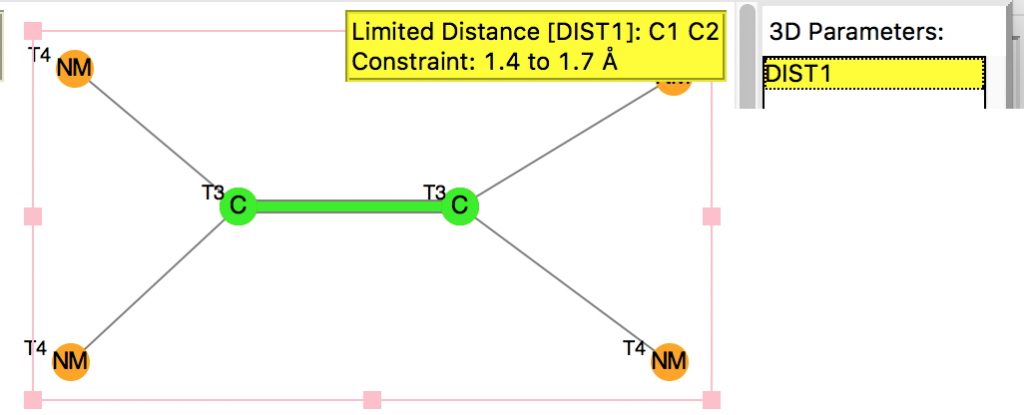
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds. The query restricts the search to each carbon having just two non-metallic substituents. To avoid conjugation with these, they each are 4-coordinated; the carbons themselves are three-coordinated.
In an earlier post, I searched for small C-C-C angles, finding one example that was also accompanied by an apparently exceptionally long C-C bond (2.18Å). But this arose from highly unusual bonding giving rise not to a single bond order but one closer to one half! How long can a “normal” (i.e single) C-C bond get, a question which has long fascinated chemists. A naive search of the CSD is not as straightforward as it seems.
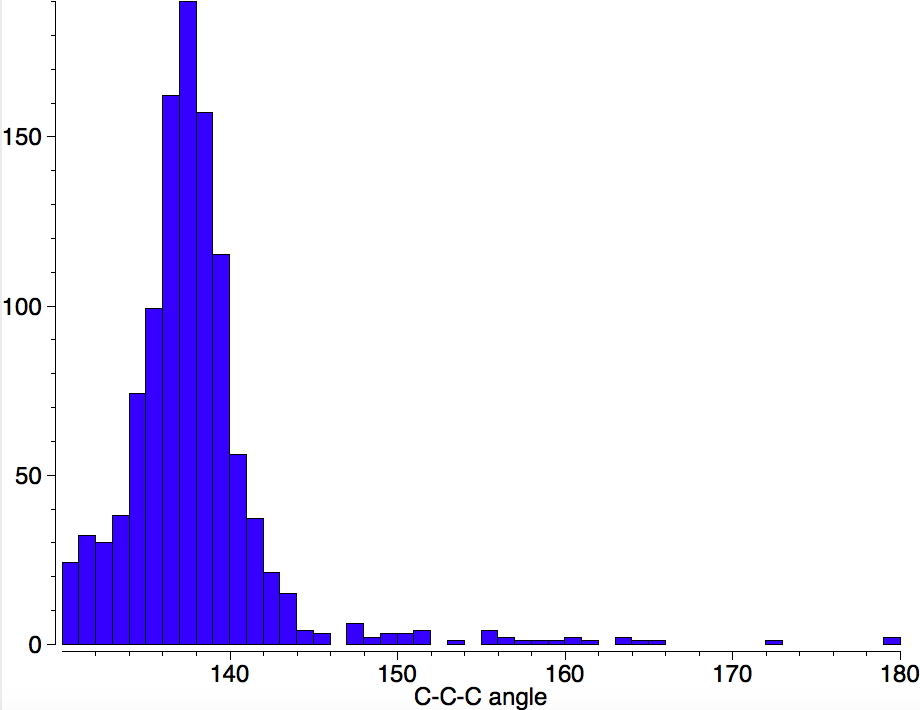
I am now inverting the previous question by asking what is the largest angle subtended at a chain of three connected 4-coordinate carbon atoms ? Let’s see if further interesting chemistry can be unearthed. Specifying only angles > 130°, the following distribution is obtained.
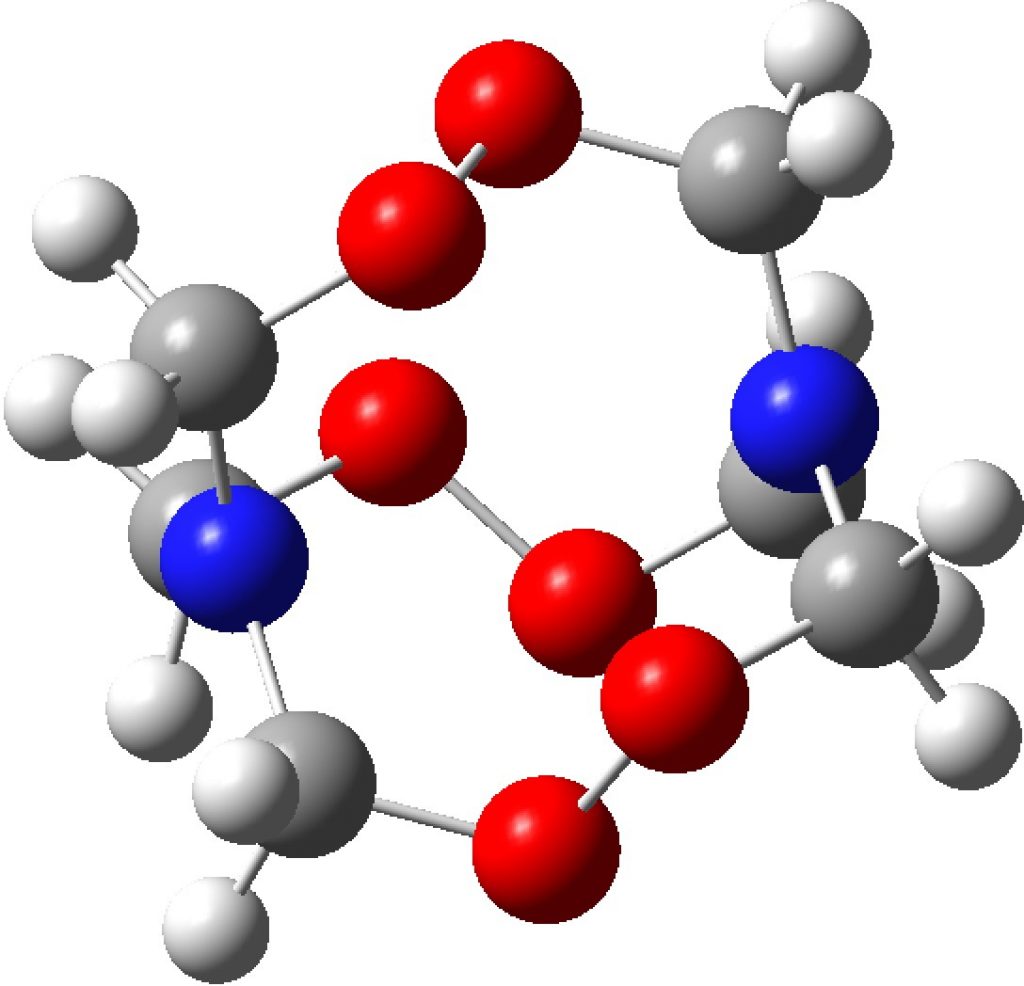
Compounds with O-O bonds often have weird properties. For example, artemisinin, which has some fascinating stereoelectronics. Here is another such, recently in the news and known as HMTD (hexamethylene triperoxide diamine). The crystal structure was reported some time ago[cite]10.1021/jp0123841[/cite] and the article included an inspection of the computed wavefunction.