Homoaromaticity is a special case of aromaticity in which π-conjugation is interrupted by a single sp 3 hybridized carbon atom (it is sometimes referred to as a suspended π-bond with no underlying σ-foundation). But consider the carbene shown below. This example comes from a recently published article[cite]10.1021/ja407116e[/cite] which was highlighted on Steve Bachrach’s blog.
Messaggi di Rogue Scholar
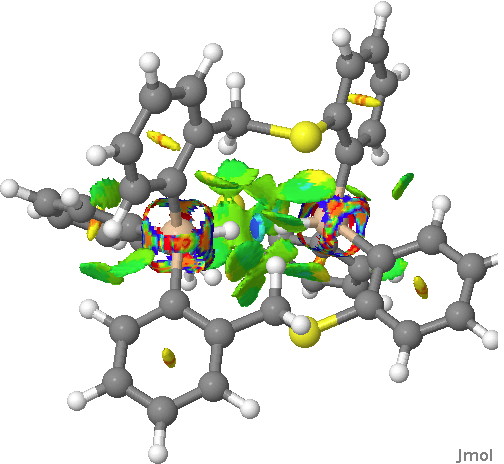
This is a continuation of the discussion started on Steve Bachrach’s blog about a molecule with a very short H…H interaction involving two Si-H groups with enforced proximity. It had been inferred from the X-ray structure[cite]10.1021/ja407398w[/cite] that the H…H distance was in the region of 1.50Å. It’s that cis-butene all over again! So is that H…H region a bond? Is it attractive or repulsive? Go read Steve’s blog first.
The best known example of the gauche effect is 1,2-difluoroethane, which exhibits a relatively small preference of ~0.5 kcal/mol for this conformer over the anti orientation, which is also a minimum. But FSSF, which I discussed in the previous post, beats this hands down!
Paul Schleyer sent me an email about a pattern he had spotted, between my post on F 3 SSF and some work he and Michael Mauksch had done 13 years ago with the intriguing title “ Demonstration of Chiral Enantiomerization in a Four-Atom Molecule ”.[cite]http://doi.org/d8g2nw[/cite] Let me explain the connection, but also to follow-up further on what I discovered in that post and how a new connection evolved.
I do go on rather a lot about enabling or hyper-activating[cite]10.1039/P29950000007[/cite] data. So do others[cite]10.1038/nj7461-243a[/cite]. Why is sharing data important? Reproducibility is a cornerstone in science, To achieve this, it is important that scientific research be open and transparent. Openly available research data is central to achieving this.
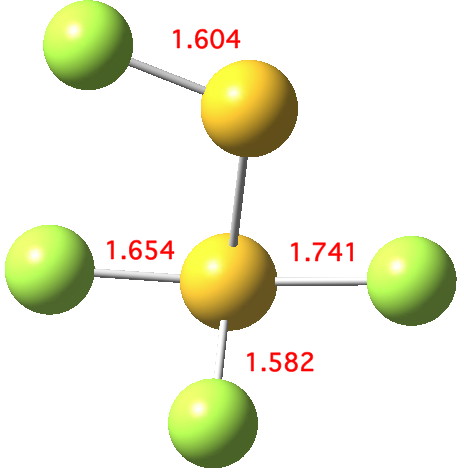
Andy Extance at the Chemistry World blog has picked up on a fascinating article[cite]10.1021/jz401578h[/cite] on the dimer of SF 2 . This molecule has three F atoms on one S, and only one on the other; FSSF 3 . But all four S-F bonds are of different length.

We have been experimenting with full-colour 3D printing of molecular objects. I thought I might here share some of our observations. Firstly, I list the software used: Crystal structures as sources of ball&stick models ( e.g. the CCDC database). Gaussian style cube files for sources of wavefunctions.
The ultimate reduction in size for an engineer is to a single molecule. It’s been done for a car; now it has been reported for the pixel (picture-element).[cite]10.1021/ja404256s[/cite] The molecule above (X=O, NR, R=aryl, etc) has been shown to be capable of acting as a molecular pixel.
Valence shell electron pair repulsion theory is a simple way of rationalising the shapes of many compounds in which a main group element is surrounded by ligands. ClF 3 is a good illustration of this theory.
This potential example of a molecule on the edge of chaos was suggested to me by a student (thanks Stephen!), originating from an inorganic tutorial. It represents a class of Mo-complex ligated by two dithiocarbamate ligands and two aryl nitrene ligands (Ar-N:). I focus on two specific examples[cite]10.1039/A907382E[/cite], where R=R’ = H or Me, with crystal structures available for both.