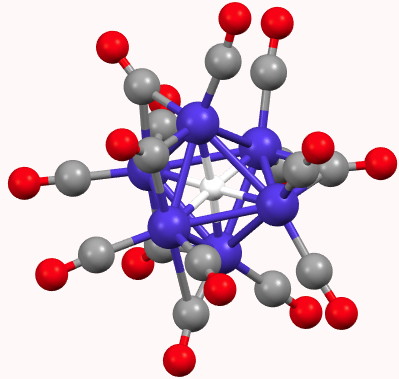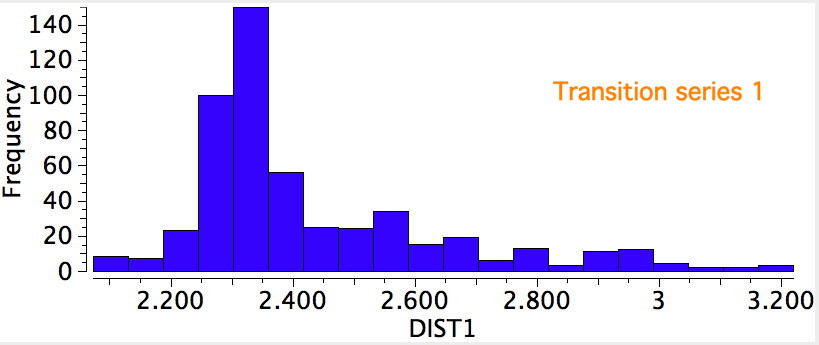
I noted previously that some 8-ring cyclic compounds could exist in either a planar-aromatic or a non-planar-non-aromatic mode, the mode being determined by apparently quite small changes in a ring substituent. Hunting for other examples of such chemistry on the edge, I did a search of the Cambridge crystal database for metal sulfides.