Students learning organic chemistry are often asked in examinations and tutorials to devise the mechanisms (as represented by curly arrows) for the core corpus of important reactions, with the purpose of learning skills that allow them to go on to improvise mechanisms for new reactions.
Messaggi di Rogue Scholar
Previously on the kinetic isotope effects for the Baeyer-Villiger reaction, I was discussing whether a realistic computed model could be constructed for the mechanism. The measured KIE or kinetic isotope effects (along with the approximate rate of the reaction) were to be our reality check.
The concept of a “ hidden intermediate ” in a reaction pathway has been promoted by Dieter Cremer[cite]10.1021/ar900013p[/cite] and much invoked on this blog. When I used this term in a recent article of ours[cite]10.1021/jo401146k[/cite], a referee tried to object, saying it was not in common use in chemistry. The term clearly has an image problem.
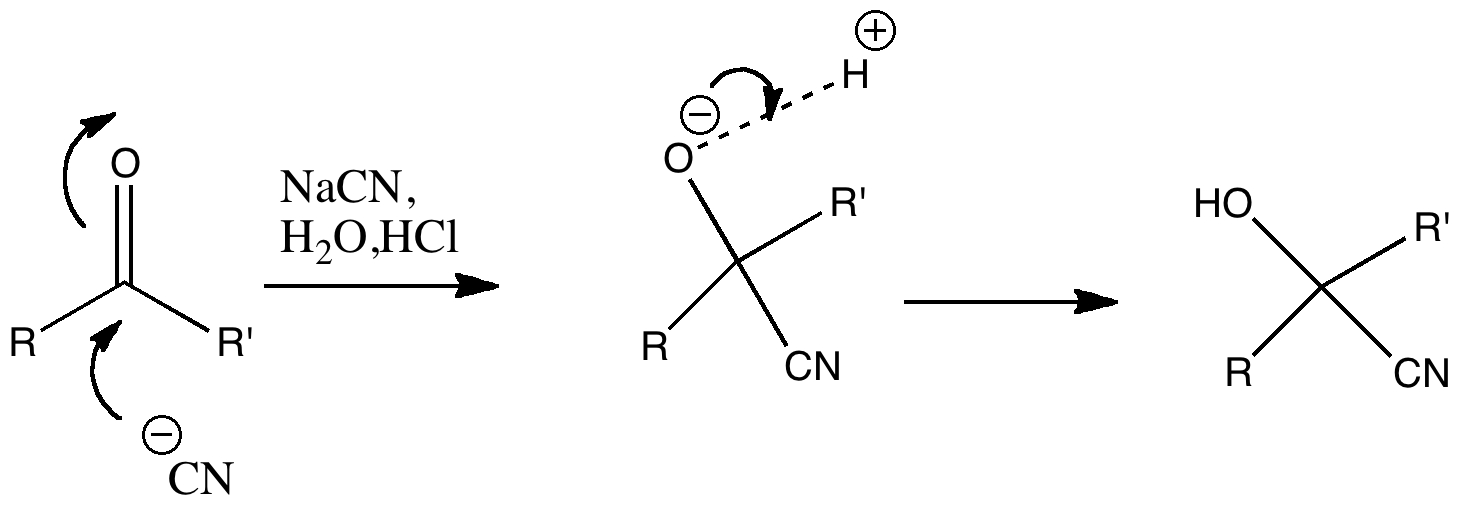
Nucleophilic addition of cyanide to a ketone or aldehyde is a standard reaction for introductory organic chemistry. But is all as it seems? The reaction is often represented as below, and this seems simple enough. Cyanohydrin formation. But attention to detail suggests that, HCN being a weak acid, there will be only a very small concentration of cyanide anion in the presence of HCl.
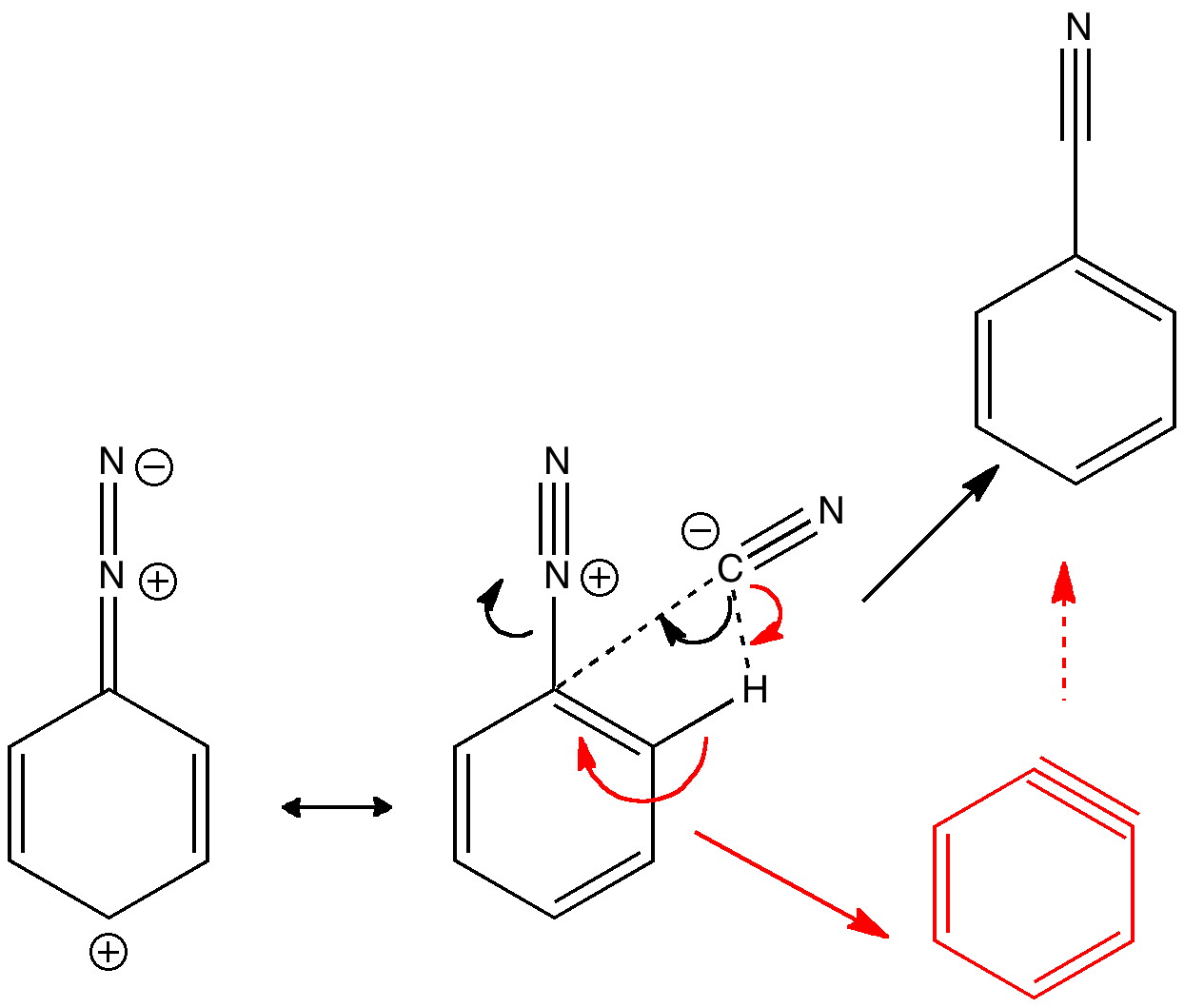
Janus was the mythological Roman god depicted as having two heads facing opposite directions, looking simultaneously into the past and the future. Some of the most ancient ( i.e. 19th century) known reactions can be considered part of a chemical mythology; perhaps it is time for a Janus-like look into their future. Reaction of the diazonium cation with cyanide.