Another post inspired by a comment on an earlier one; I had been discussing compounds of the type I.I n (n=4,6) as possible candidates for hypervalency.
Messaggi di Rogue Scholar
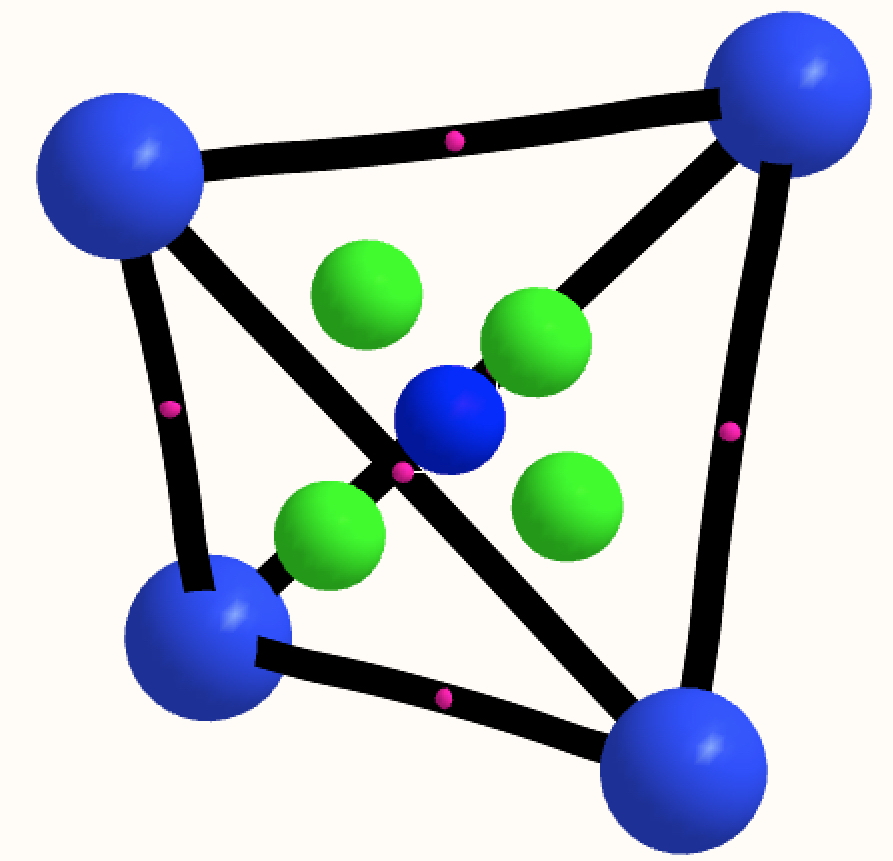
The thread thus far. The post about Na 2 He introduced the electride anionic counter-ion to Na + as corresponding topologically to a rare feature known as a non-nuclear attractor. This prompted speculation about other systems with such a feature, and the focus shifted to a tetrahedral arrangement of four hydrogen atoms as a dication, sharing a total of two valence electrons. The story now continues here.

Here is an inside peek at another one of Derek Lowe’s 250 milestones in chemistry, the polymorphism of Ritonavir .[cite]10.1023/A:1011052932607[/cite] The story in a nutshell concerns one of a pharma company’s worst nightmares;
In the previous post, I explored the mechanism for nucleophilic substitution at a silicon centre proceeding via retention of configuration involving a Berry-like pseudorotation. Here I probe an alternative route involving inversion of configuration at the Si centre.
Earlier, I constructed a possible model of hydronium hydroxide, or H3O+.OH– One way of assessing the quality of the model is to calculate the free energy difference between it and two normal water molecules and compare the result to the measured difference. Here I apply a further test of the model using isotopes.
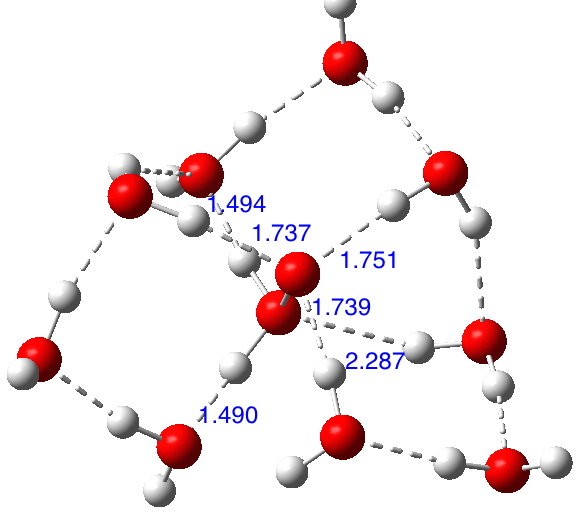
If H3N+-O– is viable compared with its tautomer H2N-OH when carrying water bridges, then why not try H2O+-O– vs HO-OH? https://orcid.org/0000-0002-8635-8390
The reduction of cinnamaldehyde by lithium aluminium hydride (LAH) was reported in a classic series of experiments[cite]10.1021/ja01197a060[/cite],[cite]10.1021/ja01202a082[/cite],[cite]10.1021/ja01190a082[/cite] dating from 1947-8. The reaction was first introduced into the organic chemistry laboratories here at Imperial College decades ago, vanished for a short period, and has recently been reintroduced again. ‡ The experiment is

I do not play poker,‡ and so I had to look up a 5-4-3-2-1(A), which Wikipedia informs me is a 5-high straight flush, also apparently known as a steel wheel. In previous posts I have suggested acids which can be ionised by (probably) 5, 4, 3 or 1 discrete water molecules in the gas phase;
Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.
The journal of chemical education can be a fertile source of ideas for undergraduate student experiments. Take this procedure for asymmetric epoxidation of an alkene.[cite]10.1021/ed077p271[/cite] When I first spotted it, I thought not only would it be interesting to do in the lab, but could be extended by incorporating some modern computational aspects as well.