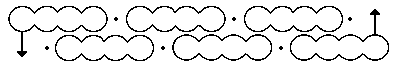
Continuing my european visits, here are two photos from Bonn. First, a word about how the representation of benzene evolved, attributed to Kekulé. The sausage formula Above is his first effort, made in 1865. The bent bond formula This one above is better, offered in 1866.