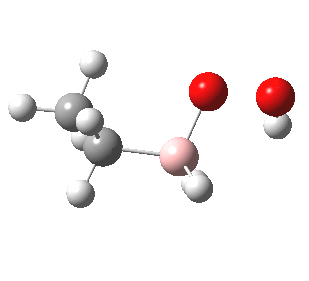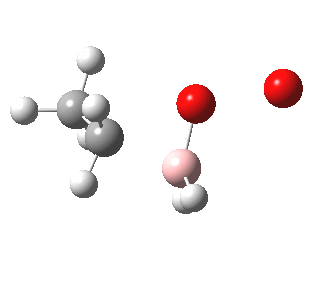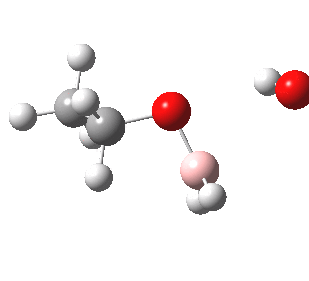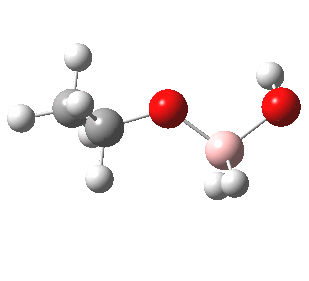The hydroboration-oxidation mechanism: An updated look.
One thing almost always leads to another in chemistry. In the last post, I described how an antiperiplanar migration could compete with an antiperiplanar elimination. This leads to the hydroboration-oxidation mechanism, the discovery of which resulted in Herbert C. Brown (at least in part) being awarded the Nobel prize in 1979.
This reaction represents a fairly steep learning curve for new students of organic chemistry. Actually, it turns out its a pretty steep learning curve for most of us.
- Firstly, it is often described as an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon (as above). Unfortunately, the mechanism is revealed as following Markovnikov, rather than being an anti example! Confusing?
- One possible way of representing the mechanism is to show the (nucleophilic) π-bond attacking the (electrophilic) boron centre of a BH3 molecule (itself presumed to be generated from a suitable reagent such as BH3.SMe2 in a pre-equilibrium step) to form a carbocation (step 1 above). The regiospecificity proceeds so as to form the most stable such carbocation (a tertiary one above). This of course follows the Markovnikov rule and not its negation. The overall reaction might be anti-Markovnikov but its mechanism is not!
- An updated interpretation (not for the faint hearted!) is nicely explained on this blog. This shows that the above “arrow pushing” mechanism, itself based on something called transition state theory, is in fact an over-simplification. Instead, it is necessary to resort to molecular dynamics[1] rather than transition state theory to explain why the boron attaches to the least substituted carbon of the alkene.
- I think I had better return to more conventional arrow pushing before I loose whatever audience I have. Step 2 above transfers a hydrogen from the boron to the carbocation to form an alkyl borane
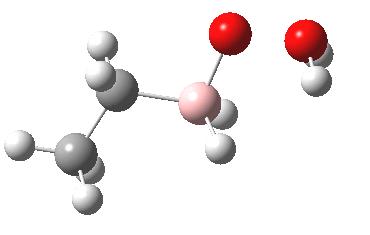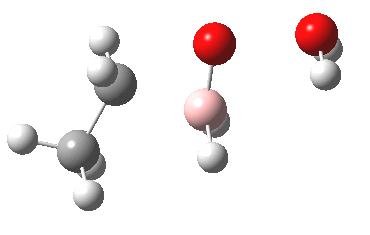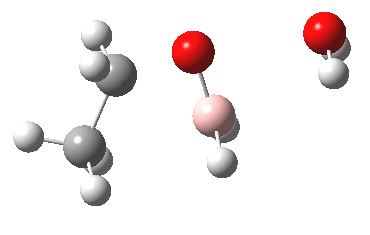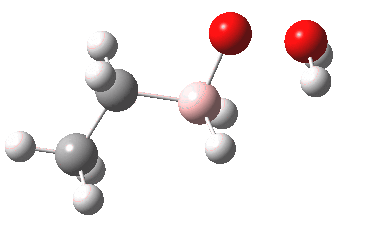
- In step 3, this species is reacted with hydrogen peroxide.
- In step 4, a proton is transferred in the resulting peroxyalkylborane. Such a step, often simply labelled as PT, is regarded as a freely available equilibrium. In other words, one can move protons around a structure to where-ever is deemed as convenient for the next step (well, within reason).
- Step 5 is the key step, and to highlight this, I have shown the arrow pushing using red arrows.
- This step now requires the donating bond (shown in blue) and the accepting (anti)bond, shown in green) to be aligned in an antiperiplanar manner. The O-O bond is a very good acceptor, the C-B bond an effective donor, quantified by the E(2) interaction energy in an NBO analysis of 35.5 kcal/mol and an overlap that looks as below:
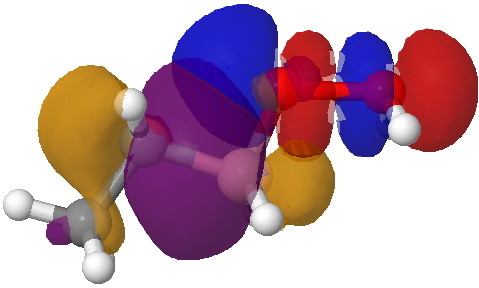
Overlap between the C-B donor and the O-O acceptor. Click for 3D.
- Note how the bonding part of the C-B bond (purple) overlaps with the blue of the O-O antibonding orbital, thus forming a new C-O bond. The (blue-red) node along the O-O leads to an entirely cleaved O-O bond, but you can see the genesis of a new B-O bond in the orange-red overlap.
- This step now requires the donating bond (shown in blue) and the accepting (anti)bond, shown in green) to be aligned in an antiperiplanar manner. The O-O bond is a very good acceptor, the C-B bond an effective donor, quantified by the E(2) interaction energy in an NBO analysis of 35.5 kcal/mol and an overlap that looks as below:
- An intrinsic reaction coordinate computed for the reaction is shown below. Note how the evicted water molecule changes direction at the end and makes a bee-line for the boron atom. The end result of this reversal is of course boric acid.
As often happens, it is worth taking a look at a tradition text-book mechanism to see what a modern slant might give it.
POSTSCRIPT: You will notice from the comments on this post below, the observation that the hydroboration-oxidation reaction is normally carried out in basic solution. I have therefore repeated the calculations using a deprotonated starting point (ωB97XD/6-311+G(d,p)/SCRF=water) as shown below.
This is best viewed as below, showing both the energy and the energy gradients as a function of the proceeding reaction.
- Note first the barrier to the migration, which is ~30 kcal/mol. This is because OH– is an inferior leaving group to H2O (for which the barrier is ~2 kcal/mol). This would make it a very slow reaction at room temperature.
- Notice how the first prominent action is a rotation of the OH group.
- The O-O bond starts to break before the C-B bond starts to migrate
- At an IRC of +6, a second feature appears, which is the reversal of the trajectory of the evicted OH group in re-attaching itself to the boron (as before).
References
- Y. Oyola, and D.A. Singleton, "Dynamics and the Failure of Transition State Theory in Alkene Hydroboration", Journal of the American Chemical Society, vol. 131, pp. 3130-3131, 2009. https://doi.org/10.1021/ja807666d
Additional details
Description
One thing almost always leads to another in chemistry. In the last post, I described how an antiperiplanar migration could compete with an antiperiplanar elimination. This leads to the hydroboration-oxidation mechanism, the discovery of which resulted in Herbert C. Brown (at least in part) being awarded the Nobel prize in 1979.
Identifiers
- UUID
- b6ae3b57-940d-4538-ac6c-2de4a87329cf
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=6315
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=6315
Dates
- Issued
-
2012-02-26T21:28:01
- Updated
-
2012-02-28T09:50:58