Hydrogen bonds: carbon as an acceptor rather than as a donor?
A hydrogen bond donor is considered as an electronegative element carrying a hydrogen that is accepted by an atom carrying a lone pair of electrons, as in X:…H-Y where X: is the acceptor and H-Y the donor. Wikipedia asserts that carbon can act as a donor, as we saw in the post on the incredible chloride cage, where six Cl**:**…H-C interactions trapped the chloride ion inside the cage. This led me to ask how many examples are there of carbon as an acceptor rather than as a donor?
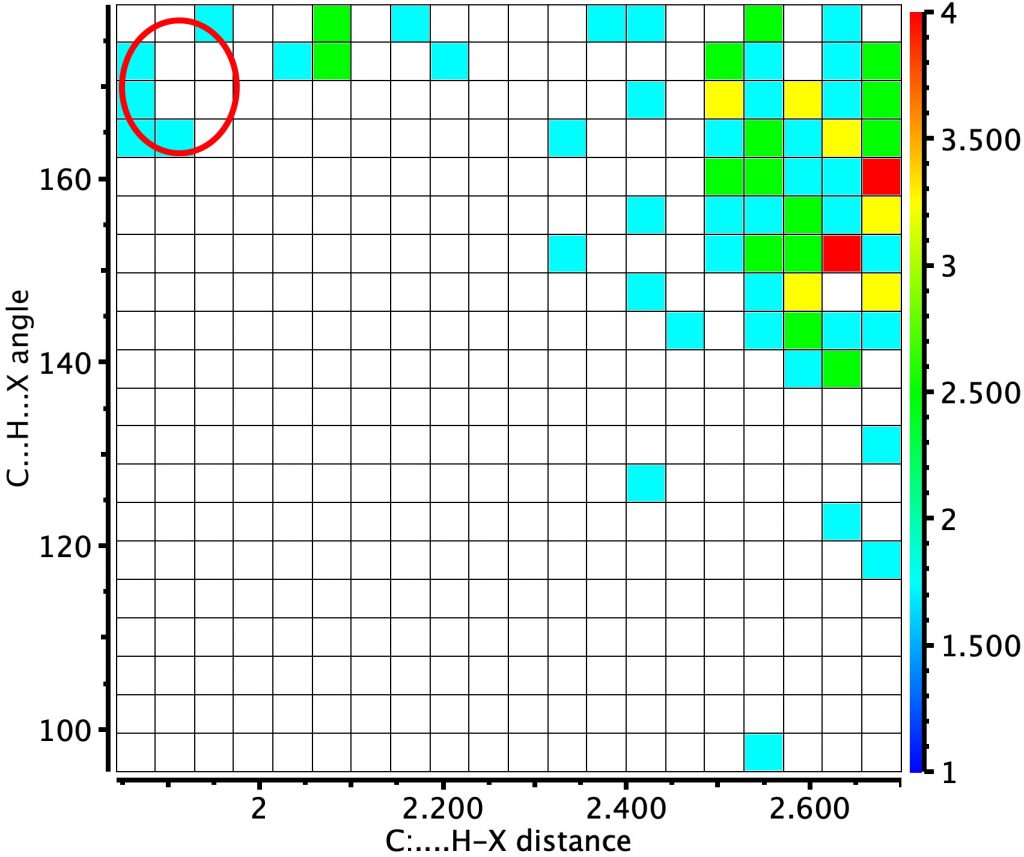
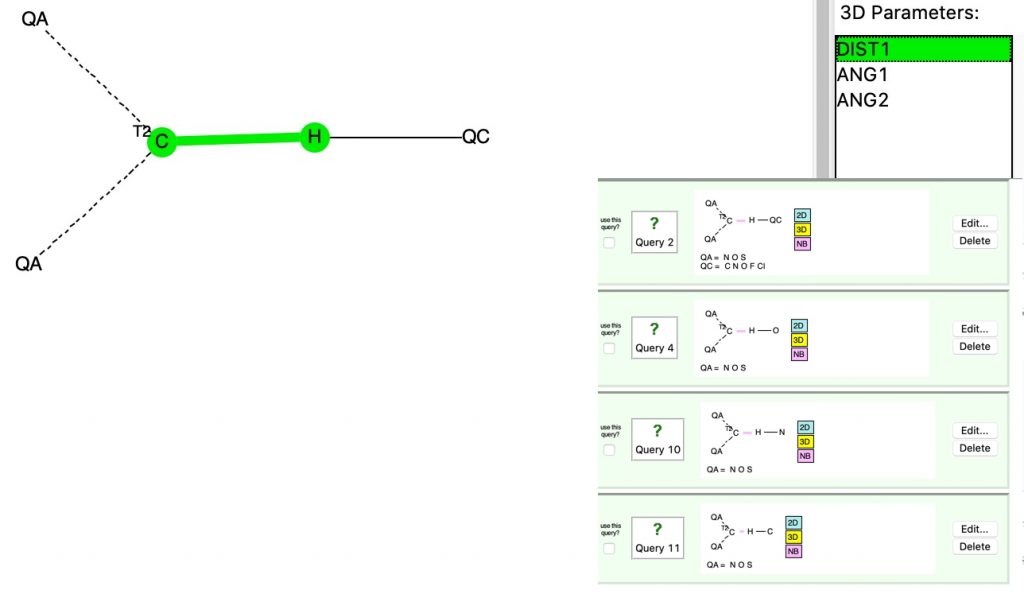
The basic query is constructed as above: in order to act as an acceptor, the carbon must bear a lone pair of electrons, of which a carbene is one example (Query, see DOI: 10.14469/hpc/6531). Thus we have QA=O,C,S and the central atom has only two connected atoms. When QC is any of C,N,O,F,Cl, we get the following result. The red circle corresponds to QC=O.
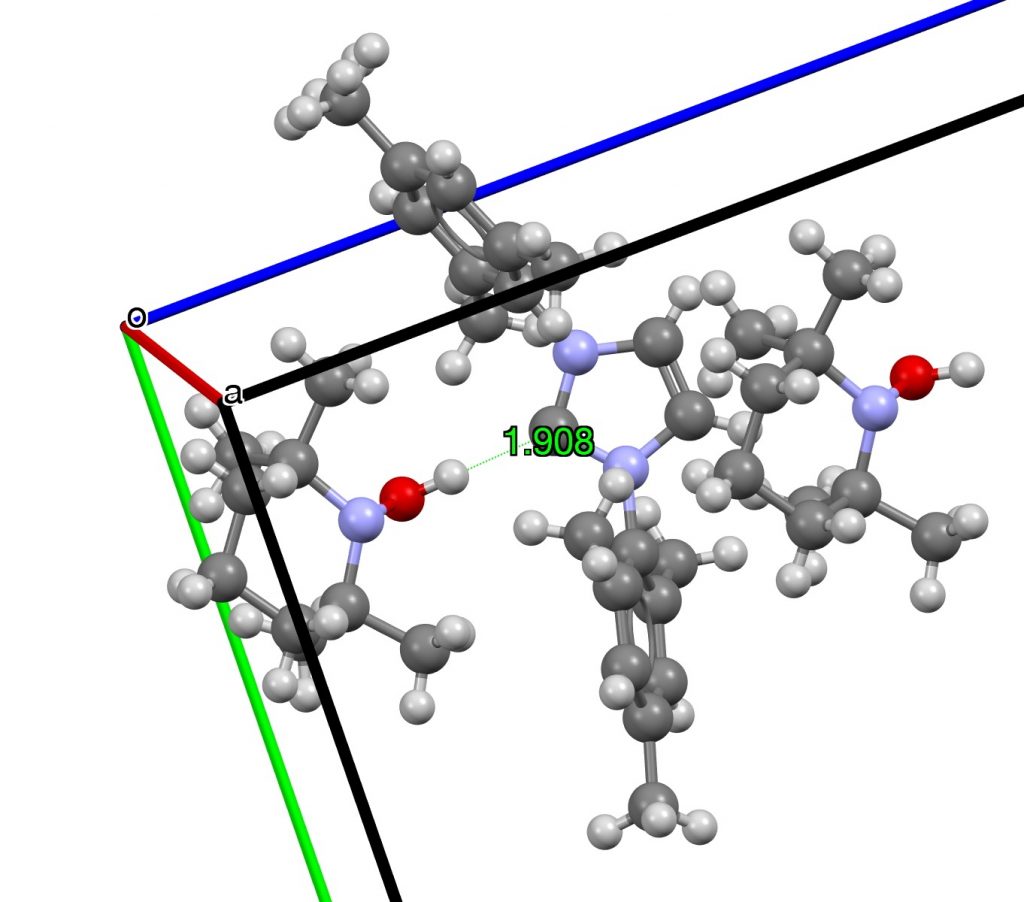
The shortest example of these is shown below, with a C**:**…HO distance of 1.91Å nominal but about 0.1Å shorter if corrected for the over-short H-O bond (Data DOI: 10.5517/ccvm80q)[cite]10.1039/c0ob00999g[/cite]
Click image to view 3D
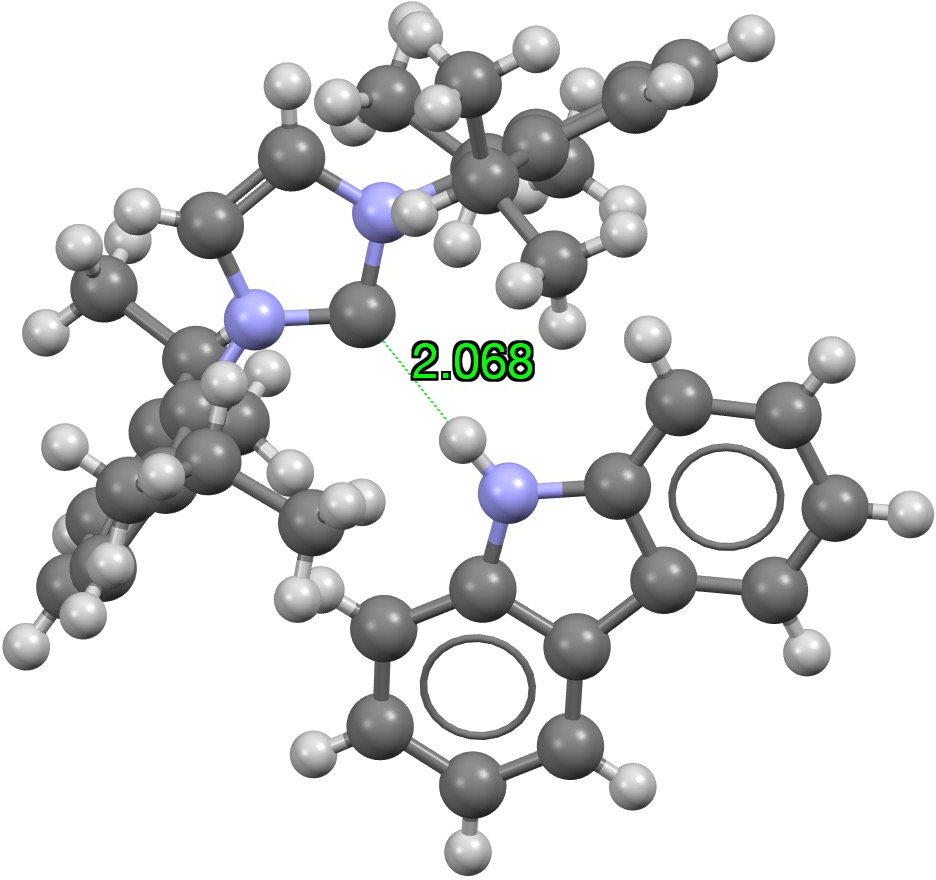
The shortest C**:**…HN example is shown below with a distance of 2.07Å (DOI: 10.5517/ccdc.csd.cc21tzql)[cite]10.1021/jacs.9b04864[/cite]
 {.size-large
.wp-image-21732 <img="" decoding="async"
aria-describedby="caption-attachment-21739"
onclick="jmolApplet([450,450],'load
wp-content/uploads/2019/12/HOKSUY.mol;measure 3 67;spin 3;','c2');"
width="450"
srcset="https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1.jpg
936w,
https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1-300x282.jpg
300w,
https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1-768x722.jpg
768w" sizes="(max-width: 936px) 100vw, 936px"}
{.size-large
.wp-image-21732 <img="" decoding="async"
aria-describedby="caption-attachment-21739"
onclick="jmolApplet([450,450],'load
wp-content/uploads/2019/12/HOKSUY.mol;measure 3 67;spin 3;','c2');"
width="450"
srcset="https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1.jpg
936w,
https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1-300x282.jpg
300w,
https://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2019/12/HOKSUY-1-768x722.jpg
768w" sizes="(max-width: 936px) 100vw, 936px"}
Click on image to view 3D model
Finally C**:**…HC, of which there are many in the region of 2.5Å, with the shortest example being 2.17Å[cite]10.1016/j.jorganchem.2006.03.018[/cite]
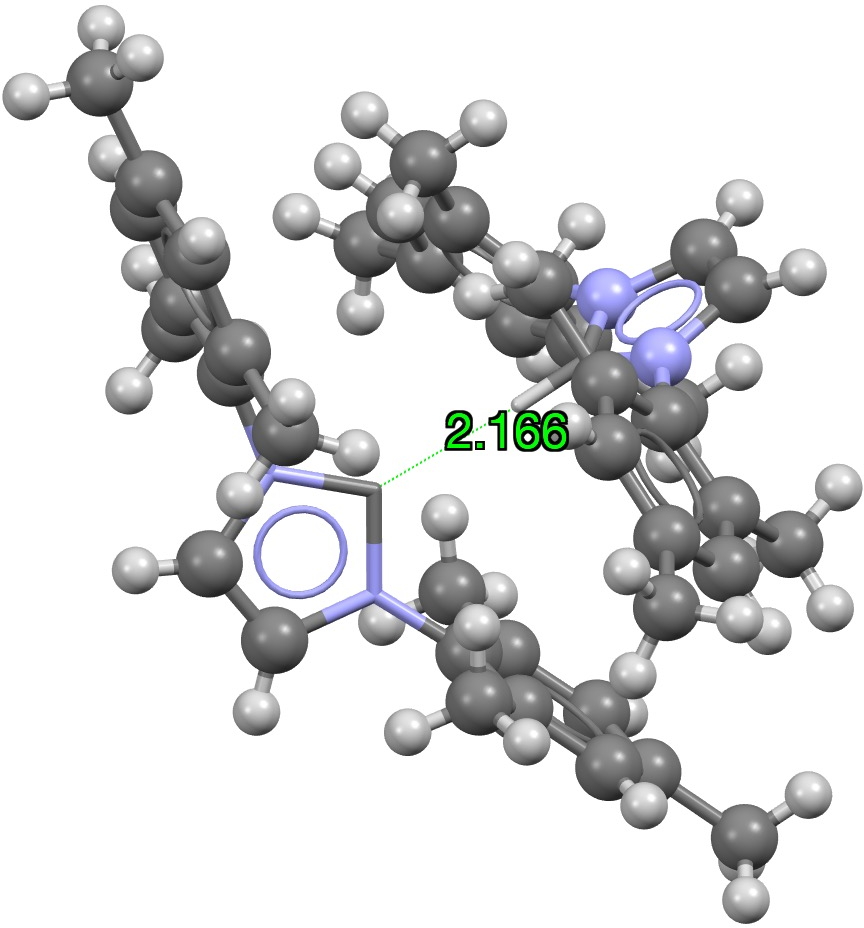
Click on image to view 3D model
To round this off, N≡C**:**…HX, of which a nice example is (DOI: 10.5517/ccs7whc)[cite]10.1016/j.phytochem.2010.09.004[/cite]
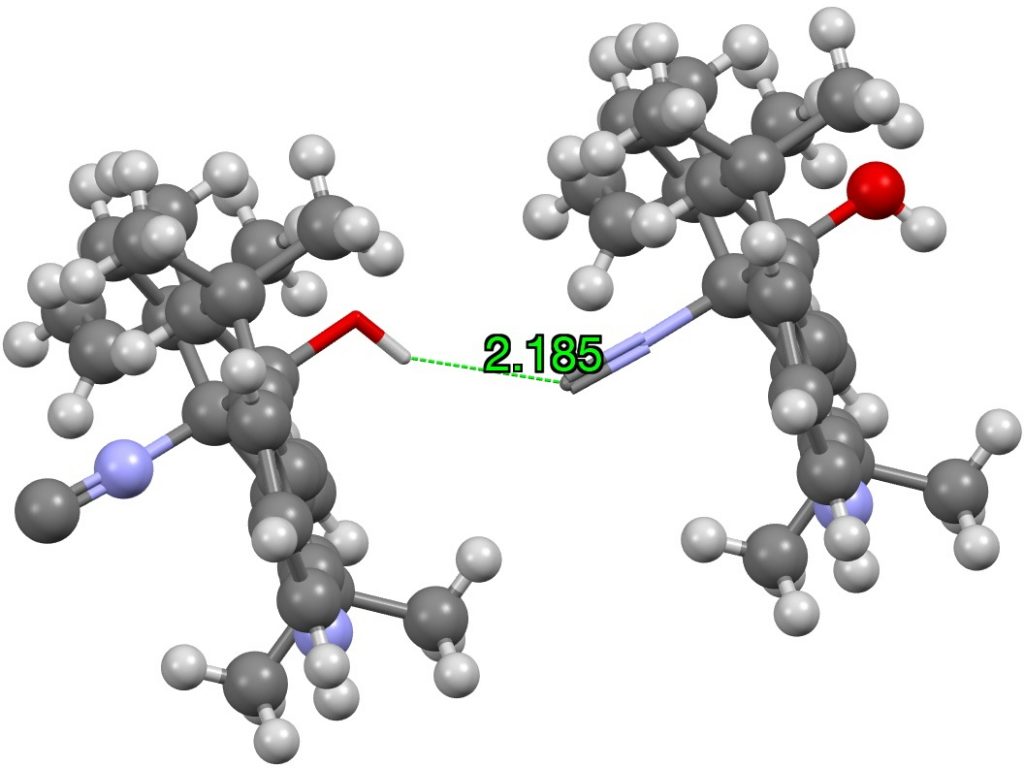
Click on image for 3D model
From which we conclude that carbon as a hydrogen bond acceptor exhibits a diversity of forms, often with surprisingly short distances! I guess the wikipedia article needs updating.
Additional details
Description
A hydrogen bond donor is considered as an electronegative element carrying a hydrogen that is accepted by an atom carrying a lone pair of electrons, as in X:…H-Y where X: is the acceptor and H-Y the donor. Wikipedia asserts that carbon can act as a donor, as we saw in the post on the incredible chloride cage, where six Cl**:**…H-C interactions trapped the chloride ion inside the cage.
Identifiers
- UUID
- 569a1210-6bdb-44a8-a164-205d1ac217b7
- GUID
- https://www.ch.imperial.ac.uk/rzepa/blog/?p=21726
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=21726
Dates
- Issued
-
2019-12-23T19:24:34
- Updated
-
2019-12-23T21:29:14
References
- Giffin, N. A., Makramalla, M., Hendsbee, A. D., Robertson, K. N., Sherren, C., Pye, C. C., Masuda, J. D., & Clyburne, J. A. C. (2011). Anhydrous TEMPO-H: reactions of a good hydrogen atom donor with low-valent carbon centres. Organic & Biomolecular Chemistry, 9(10), 3672. https://doi.org/10.1039/c0ob00999g
- Kieser, J. M., Kinney, Z. J., Gaffen, J. R., Evariste, S., Harrison, A. M., Rheingold, A. L., & Protasiewicz, J. D. (2019). Three Ways Isolable Carbenes Can Modulate Emission of NH-Containing Fluorophores. Journal of the American Chemical Society, 141(30), 12055–12063. https://doi.org/10.1021/jacs.9b04864
- Jones, C., Mills, D. P., & Rose, R. P. (2006). Oxidative addition of an imidazolium cation to an anionic gallium(I) N-heterocyclic carbene analogue: Synthesis and characterisation of novel gallium hydride complexes. Journal of Organometallic Chemistry, 691(13), 3060–3064. https://doi.org/10.1016/j.jorganchem.2006.03.018
- Mo, S., Krunic, A., Santarsiero, B. D., Franzblau, S. G., & Orjala, J. (2010). Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry, 71(17-18), 2116–2123. https://doi.org/10.1016/j.phytochem.2010.09.004