Atropisomerism in Taxol. An apparently simple bond rotation?
My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule. Introductory chemistry tells us that the barrier for rotation about such single bonds is low (i.e. fast at room temperature). But is that true here?
It looks fairly obvious that the process above must involve twisting about two bonds, presumably the red and blue ones. If that assumption is correct, one can move on to ask whether the transition state involves a synchronous twisting about each of them, or whether the two bonds twist at different rates (asynchronously). As transition states go, it is akin to asking whether the two bonds that form in e.g. a Diels Alder cycloaddition reaction form at the same rate (a concerted synchronous reaction) or at different rates (a concerted asynchronous reaction) or even where one bond is complete before the other has started to form (a non-concerted reaction). At least in that situation, we are pretty sure that the reaction coordinate is appropriately defined by the two C-C bonds. So on to atropisomerism. If you click on the above diagram, you will get an animation of the transition mode for the atropisomeric process in this taxol-like molecule. Look at it carefully and decide for yourself where the rotation is actually taking place.
The next diagram is the IRC (intrinsic reaction coordinate) for the process. Unlike the transition mode which is a harmonic motion, the IRC can be anharmonic. This in turn means the reaction coordinate can evolve, and mutate as the reaction proceeds!
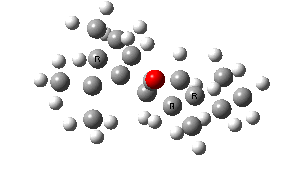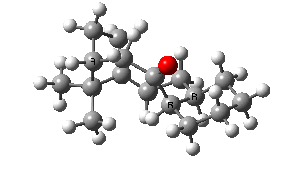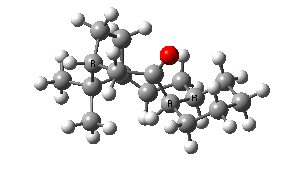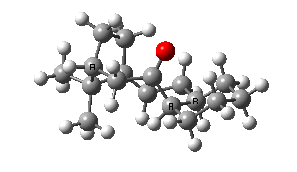
IRC for taxol atropisomerism
- The process occurs in stages. Firstly, the carbonyl group rotates (red and blue bonds)
- This then stops, and the methylene group starts to rotate (green and red bonds). This is the motion that is occurring at the actual transition state.
- The methylene group rotation then slows down and the carbonyl group starts up again (red and blue bonds).
| IRC | Gradients of IRC |
|---|---|
As reactions go therefore, this one is defined by quite a complex reaction coordinate, most definitely not a simple twist about the two most obvious single bonds.
Additional details
Description
My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule.
Identifiers
- UUID
- 77705f42-5def-4163-ae53-3cd32080f7a0
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=5345
- URL
- https://www.ch.imperial.ac.uk/rzepa/blog/?p=5345
Dates
- Issued
-
2011-11-01T10:30:39
- Updated
-
2011-11-28T14:12:49
