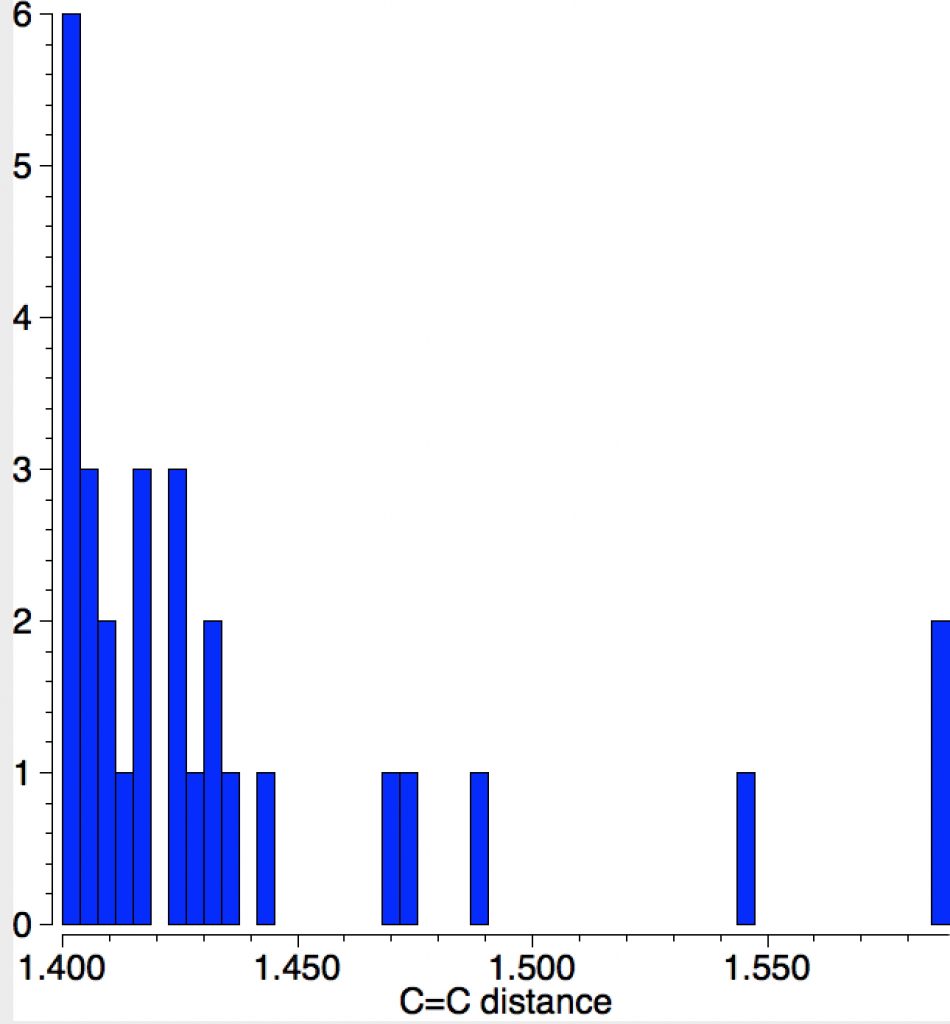
Long C=C bonds.
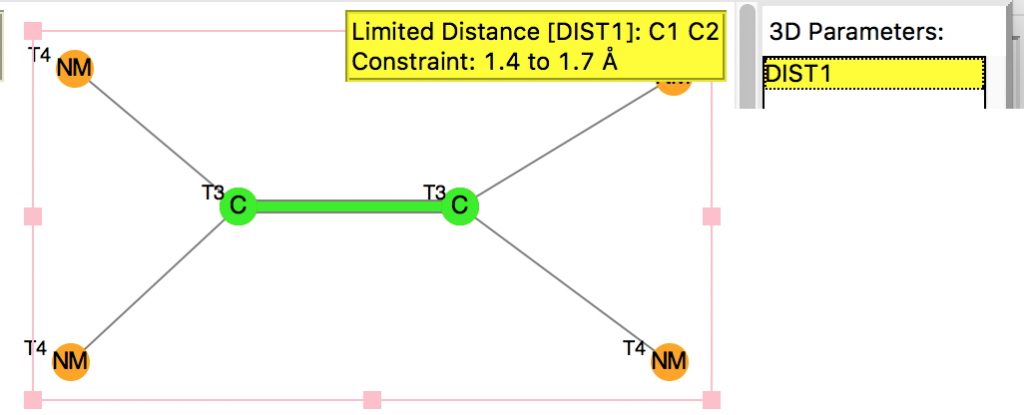
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds.

The query restricts the search to each carbon having just two non-metallic substituents. To avoid conjugation with these, they each are 4-coordinated; the carbons themselves are three-coordinated. Further constraints are the usual no disorder, no errors and R < 0.1 and the C=C distance > 1.4Å (the standard value is ~1.32-1.34Å). The search query is deposited as DOI: 10.14469/hpc/1959[cite]10.14469/hpc/1959[/cite]
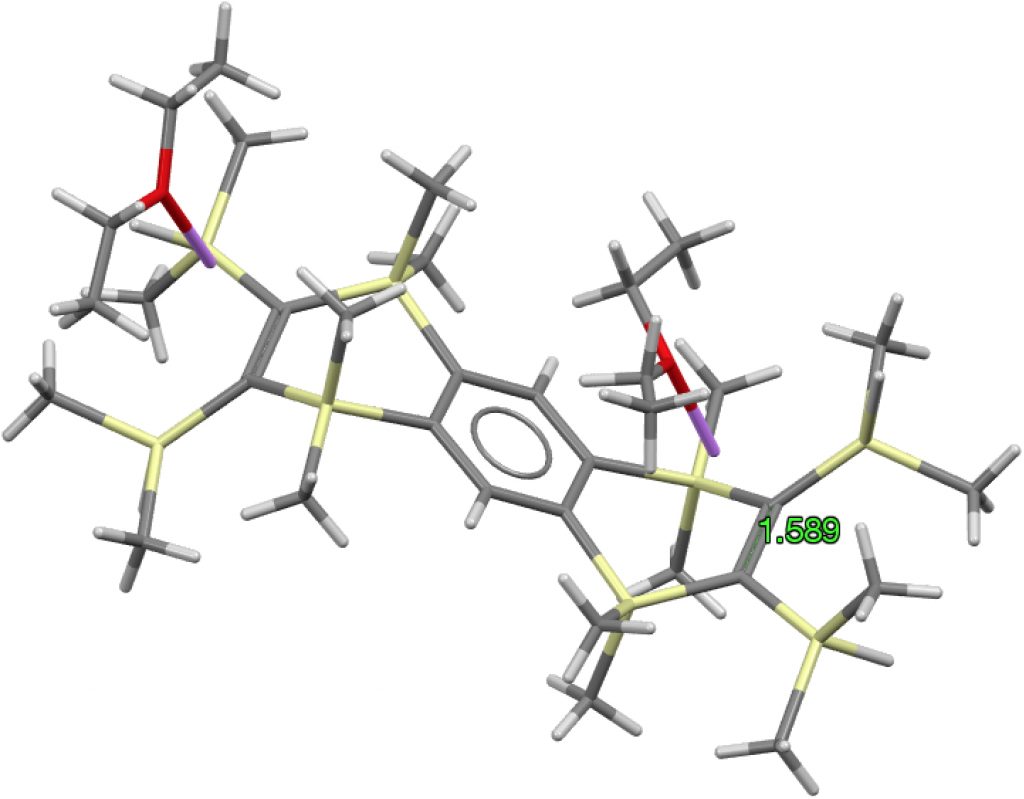
The apparent longest example is LIRVEN, DOI: 10.5517/CC4R2MK[cite]10.5517/CC4R2MK[/cite] with a value of 1.589Å, longer than most C-C single bonds! Closer inspection reveals the presence of lithium cations, and so the molecule bearing the C=C bond must sustain two negative charges. So this apparent C=C bond is in fact anionic, with one electron going into each of the π* orbitals, thus lengthening the CC bond.‡ Not a true example of a neutral C=C bond[cite]10.1016/S1387-7003(99)00136-7[/cite] but it now becomes interesting for what its spin state might be. Is it a biradical or a triplet for example? One to be investigated further I fancy! Another example of this type is QUKCEE[cite]10.1246/bcsj.73.1461[/cite]
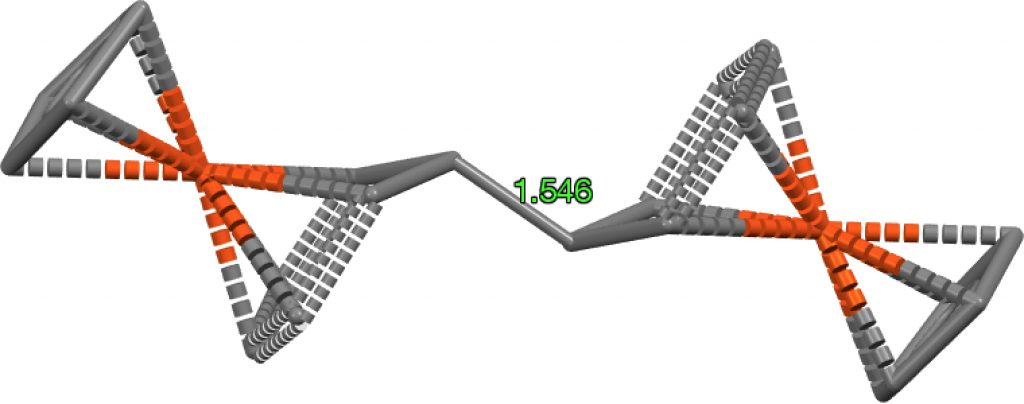
This next FAZWIM has a C=C length of 1.546Å. It is an old structure
(1986), and comes without attached hydrogen atoms. Although drawn with
no hydrogens on the central C=C bond, the length suggests this molecule
is simply mis-assigned.†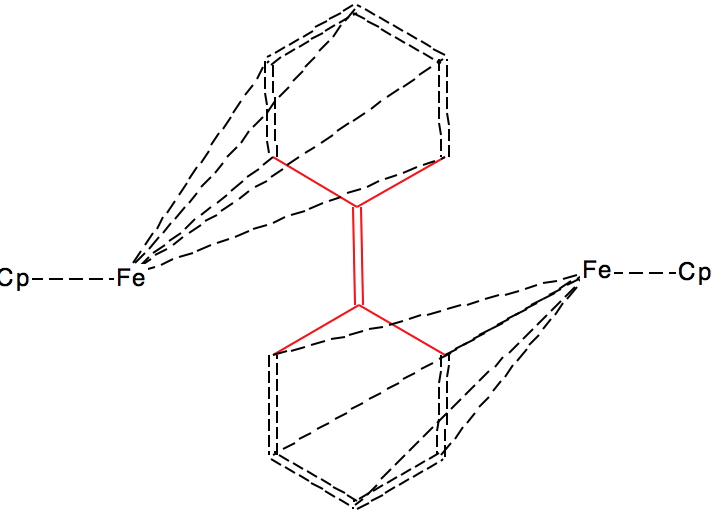
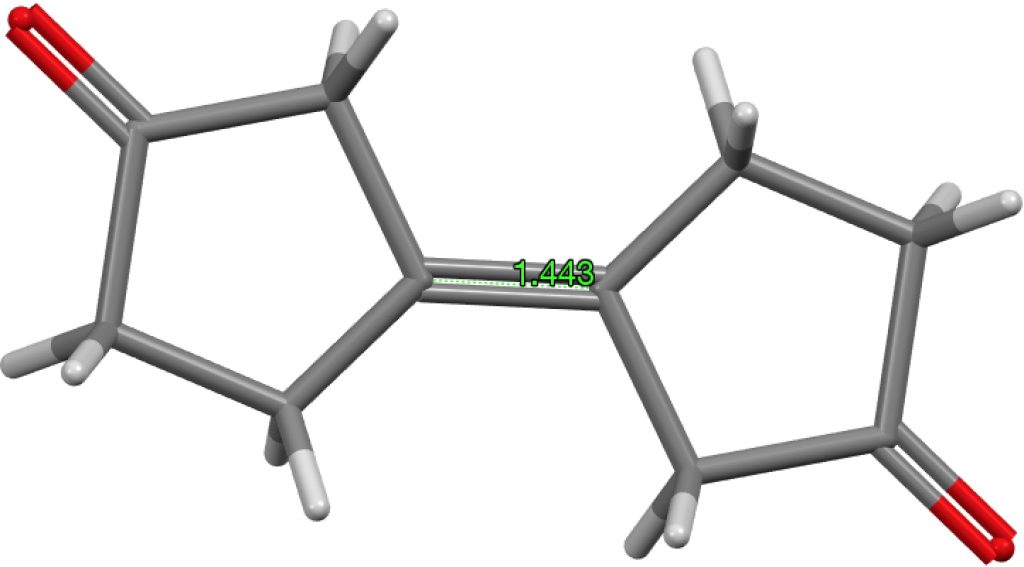
The final example I will highlight is pretty ordinary looking and published in 2016 as a private communication; ALOVOO, DOI: 10.5517/CCDC.CSD.CC1LJSWS[cite]10.5517/CCDC.CSD.CC1LJSWS[/cite] with a C=C length of 1.443Å. Again no obvious reason for the bond to be longer than normal.‡†
In hunting for such unusual deviations from the norm, the most obvious explanation is normally some anomaly in the crystallographic analysis. Although the CSD (crystal structure database) is a very heavily curated resource, it seems unlikely that each deposition would be carefully inspected for its chemistry, and this must be our task here. But such anomalies can themselves point to interesting or unusual chemistry, which in turn can be subjected to quantum computation to see if either the unusual value can be replicated or other reasons identified. In this case, this exercise can been conducted by a human, but one can easily envisage the entire process being automated on a far larger scale. The future?
‡ In fact the stoichiometry shows each "double bond" is actually a di-anion, with two electrons entering each of the the π* orbitals.
†A calculation on the singlet state for the structure as drawn (ωB97XD/Def2-TZVPP, DOI: 10.14469/hpc/1960) gives a bond length of 1.342Å, i.e. that expected for a double bond. The triplet state is similar in energy, but with a much longer central bond length of 1.476Å, DOI: 10.14469/hpc/1962 but the geometry at the carbons is planar and not bent as shown above. The quintet state is 1.45Å and is again planar, doi 10.14469/hpc/1963. So calculations on FAZWIM strongly suggest the structure as shown is an error.
‡†The computed value is 1.324Å, perfectly normal. DOI: 10.14469/hpc/1966[cite]10.14469/hpc/1966[/cite]
Additional details
Description
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds. The query restricts the search to each carbon having just two non-metallic substituents. To avoid conjugation with these, they each are 4-coordinated; the carbons themselves are three-coordinated.
Identifiers
- UUID
- 14133af4-2bd1-45c8-9b0c-ad03236fc9aa
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=17122
- URL
- https://www.ch.imperial.ac.uk/rzepa/blog/?p=17122
Dates
- Issued
-
2016-12-01T12:24:20
- Updated
-
2016-12-05T14:29:42
References
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Long C=C bonds [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1959
- Matsuo, T., Watanabe, H., Ichinohe, M., & Sekiguchi, A. (2000). CCDC 141348: Experimental Crystal Structure Determination [Data set]. Cambridge Crystallographic Data Centre. https://doi.org/10.5517/cc4r2mk
- Matsuo, T., Watanabe, H., Ichinohe, M., & Sekiguchi, A. (1999). Reduction of the 1,4,5,8-tetrasila-1,4,5,8-tetrahydroanthracene derivative with lithium metal. Isolation and characterization of the tetralithium salt of a tetraanion, and observation of an Si–H⋯Li+ interaction. Inorganic Chemistry Communications, 2(10), 510–512. https://doi.org/10.1016/s1387-7003(99)00136-7
- Matsuo, T., Watanabe, H., & Sekiguchi, A. (2000). A Novel Tetralithium Salt of a Tetraanion and a Dilithium Salt of a Dianion, Formed by the Reduction of the Tetrasilylethylene Moiety. Synthesis, Characterization, and Observation of an Si-H···Li+ Interaction. Bulletin of the Chemical Society of Japan, 73(7), 1461–1467. https://doi.org/10.1246/bcsj.73.1461
- Light, M. E., Bain, S., & Kilburn, J. (2016). CCDC 1475906: Experimental Crystal Structure Determination [Data set]. Cambridge Crystallographic Data Centre. https://doi.org/10.5517/ccdc.csd.cc1ljsws
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). ALOVOO [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1966
