Why is the Sharpless epoxidation enantioselective? Part 1: a simple model.
Sharpless epoxidation converts a prochiral allylic alcohol into the corresponding chiral epoxide with > 90% enantiomeric excess[cite]10.1021/jo00369a032[/cite],[cite]10.1021/jo00360a058[/cite]. Here is the first step in trying to explain how this magic is achieved.
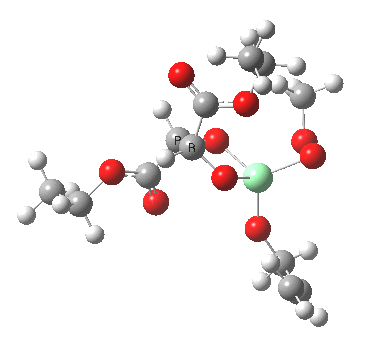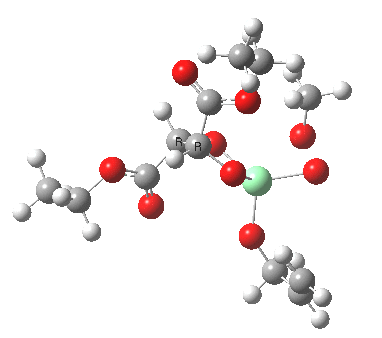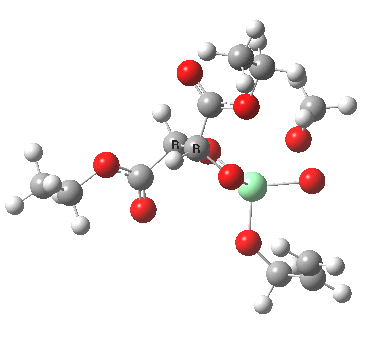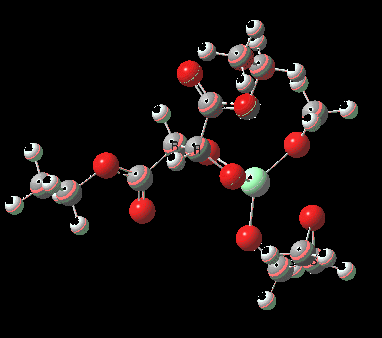
The scheme above shows how (achiral) prop-2-enol is converted using the asymmetric catalyst (R,R)-diethyl tartrate and t-butyl hydroperoxide as oxidant into the (S)-chiral epoxide. The first step is to try to construct a simple model for the reaction, and in this post I will start by using one titanium as the core of the stage on which these actors will perform. This is the mononuclear model†. One can simply envisage that a molecule of tartrate displaces two iPrOH molecules from Ti(OiPr)4 in an ester exchange to form a Ti(OiPr)2(tartrate) complex. The remaining two iso-propanols are then replaced by one molecule each of prop-2-enol and tBu-OOH. Now we have the species Ti(OOtBu)(O-CH2CH=CH2)(tartrate) as the starting point from which a transition state for oxygen transfer to the alkene to form the (S) epoxide (for R,R tartrate) can be constructed (ωB97XD/6-311G(d,p)/SCRF=dichloromethane model).
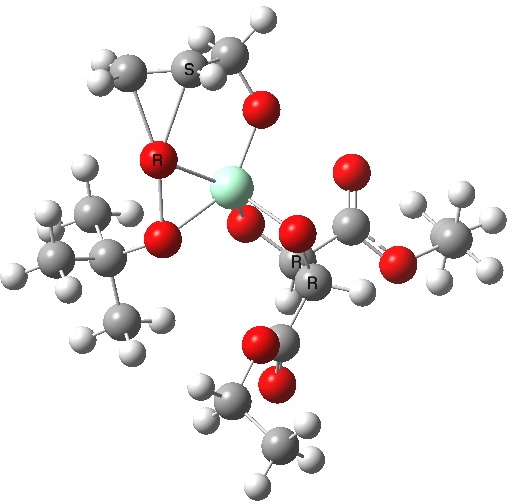
Mononuclear TS for S-epoxide. Click for 3D. |
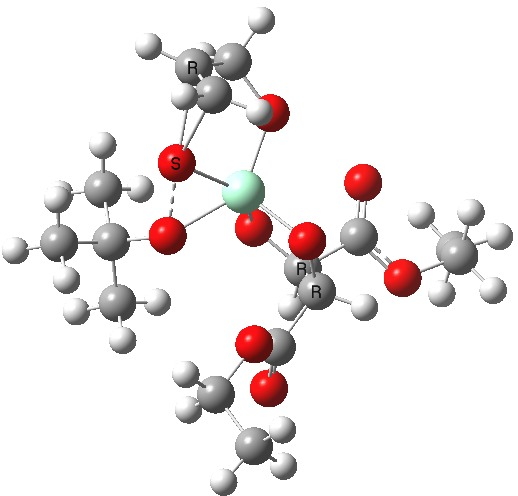
Mononuclear TS for R-epoxide. Click for 3D. |
| IRC for mononuclear model showing oxygen atom transfer | |
 |
|
The transition state leading to (S) epoxide emerges as 0.86 kcal/mol higher in ΔG‡ than the (R), contrary to the experimental result where (S) is formed with high specificity[cite]10.1021/jo00369a032[/cite]. Inspecting the model, it is clear that the allylic alcohol substrate sits in a very open pocket un-encumbered by any nearby groups (bottom right in the animation above) and so the lack of π-facial selectivity is perhaps not surprising.
To elaborate the model, I will turn to a crystal structure determined for a Ti complex bearing a t-butyl peroxy group[cite]10.1021/ja954308f[/cite], showing it to be a binuclear complex¶ (magenta arrows indicate the peroxy groups) with bridging oxygen atoms.‡
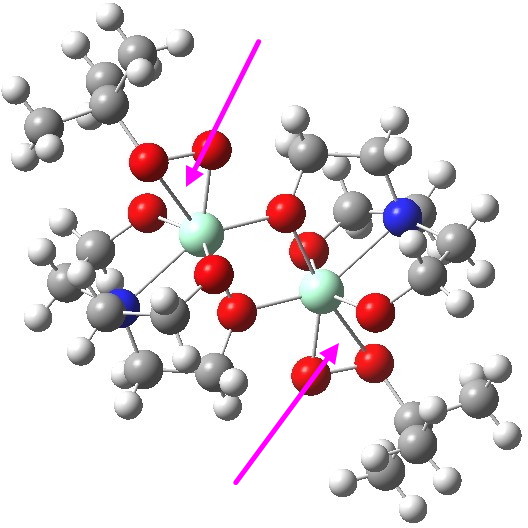
ZUKJIY. Click for 3D
In the follow-up post, we will see whether these binuclear models can do better at explaining the enantioselectivity of the Sharpless reaction.
† See this post for an example of such "single-site" catalysis using Mg or this article for an example using silver[cite]10.1002/chem.201200547[/cite].
¶A binuclear Zn catalyst with similar oxy-bridges is used to co-polymerise epoxides themselves with carbon dioxide[cite]10.1021/ma300803b[/cite]. Many such binuclear complexes are known.
‡ The other element for which a number of examples of such t-butyl peroxy bonding are known is oddly enough, lithium.[cite]10.1002/chem.200900746[/cite]
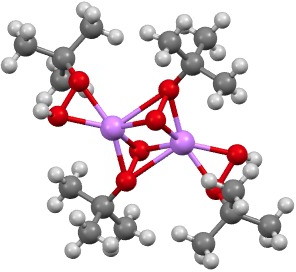
MUKVAQ. Click for 3D.
Postscript: Two lower energy conformations for the S and R transition states have been found, the latter being 1.6 kcal/mol lower in free energy.
| S | R |
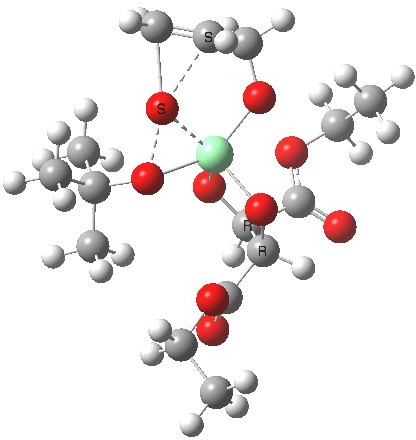 |
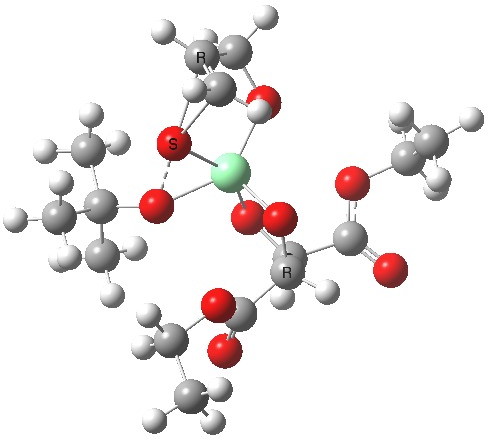 |
Additional details
Description
Sharpless epoxidation converts a prochiral allylic alcohol into the corresponding chiral epoxide with > 90% enantiomeric excess[cite]10.1021/jo00369a032[/cite],[cite]10.1021/jo00360a058[/cite]. Here is the first step in trying to explain how this magic is achieved.
Identifiers
- UUID
- 9047aaa3-4bb6-4fd3-8f2a-dfeef2ff28fd
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=8588
- URL
- https://www.ch.imperial.ac.uk/rzepa/blog/?p=8588
Dates
- Issued
-
2012-12-09T09:56:23
- Updated
-
2023-03-10T09:23:37
References
- Klunder, J. M., Ko, S. Y., & Sharpless, K. B. (1986). Asymmetric epoxidation of allyl alcohol: efficient routes to homochiral .beta.-adrenergic blocking agents. The Journal of Organic Chemistry, 51(19), 3710–3712. https://doi.org/10.1021/jo00369a032
- Hanson, R. M., & Sharpless, K. B. (1986). Procedure for the catalytic asymmetric epoxidation of allylic alcohols in the presence of molecular sieves. The Journal of Organic Chemistry, 51(10), 1922–1925. https://doi.org/10.1021/jo00360a058
- Boche, G., Möbus, K., Harms, K., & Marsch, M. (1996). [((η2-tert-Butylperoxo)titanatrane)2· 3 Dichloromethane]: X-ray Crystal Structure and Oxidation Reactions. Journal of the American Chemical Society, 118(11), 2770–2771. https://doi.org/10.1021/ja954308f
- Arbour, J. L., Rzepa, H. S., Contreras‐García, J., Adrio, L. A., Barreiro, E. M., & Hii, K. K. (Mimi) . (2012). Silver‐Catalysed Enantioselective Addition of OH and NH Bonds to Allenes: A New Model for Stereoselectivity Based on Noncovalent Interactions. Chemistry – A European Journal, 18(36), 11317–11324. https://doi.org/10.1002/chem.201200547
- Buchard, A., Jutz, F., Kember, M. R., White, A. J. P., Rzepa, H. S., & Williams, C. K. (2012). Experimental and Computational Investigation of the Mechanism of Carbon Dioxide/Cyclohexene Oxide Copolymerization Using a Dizinc Catalyst. Macromolecules, 45(17), 6781–6795. https://doi.org/10.1021/ma300803b
- Uhl, W., Reza Halvagar, M., & Claesener, M. (2009). Reducing GaH and GaC Bonds in Close Proximity to Oxidizing Peroxo Groups: Conflicting Properties in Single Molecules. Chemistry – A European Journal, 15(42), 11298–11306. https://doi.org/10.1002/chem.200900746
