The subtle effect of dispersion forces on the shapes of molecules: benzyl magnesium bromide.
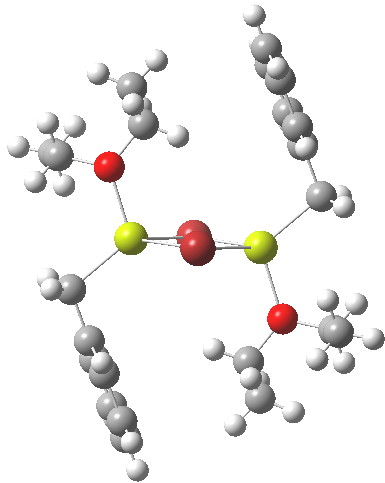
In the previous post I mentioned in passing the Grignard reagent benzyl magnesium bromide as having tetrahedral coordination at Mg. But I have now noticed, largely through spotting Steve Bachrach’s post on “Acene dimers – open or closed?” another geometric effect perhaps worthy of note, certainly one not always noted in the past; that of dispersion forces.
| crystal structure | Calc structure |
|---|---|
 Click for 3D |
 Click for 3D |
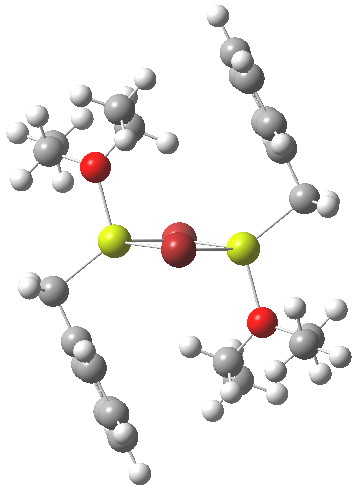
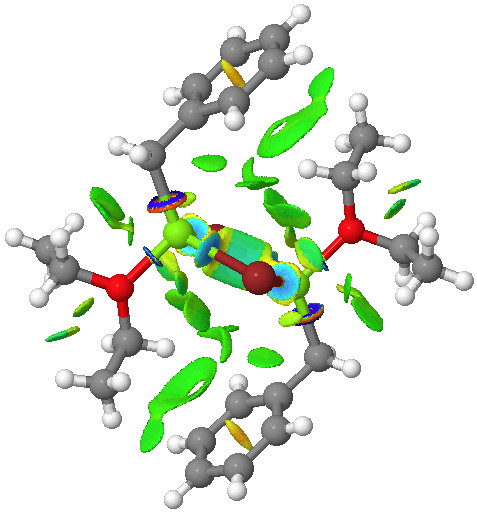
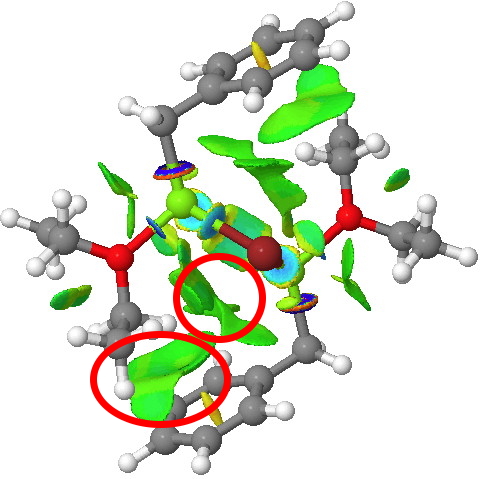
On the left is the crystal structure.[1] and on the right a ωB97Xd/6-311G(d,p) calculation, with built-in dispersion correction. If you compare the two you will find that the ethyl groups from the ether are about 0.3Å closer to the face of the phenyl group in the calculated structure. Why? Well, in the crystal structure, each dimeric Grignard unit is surrounded by adjacent units in the periodic lattice. You would think that this would have the effect of compressing the structure. Instead it is more open, and it is the isolated calculated structure that is compressed. The dispersion forces are responsible for this. In the crystal structure, the phenyl group is attracted not only to the ethyl groups but also by adjacent units in the lattice by dispersion forces. This balancing effect is absent in the calculated structure and so manifests as just an attraction between the phenyl face and the methyl groups, which pulls them together by ~0.3Å. One can see this more clearly when an NCI (non-covalent-interactions) isosurface is shown at both geometries:
| X-ray geometry[2] | Calc. geometry [3] |
|---|---|
 Click for 3D |
 Click for 3D |
The surface on the right is ringed (red) in the relevant region to show how the green NCI surface is so much larger compared to the one computed for the crystal structure. Notice by the way the strong stabilizing (blue) zone between the two Mg atoms. Whether it should be called metal-metal bonding is another issue, but it is a clear effect.
One can compare this result with NCI surfaces computed for the examples described in Steve’s blog (deriving from this article[4]). There two geometric isomers of the same molecule were described, differing only in the dispersion attractions. The top one is an open form and the NCI surface shows no features along the acene chain away from the central pivot. The second isomer shows stabilizing green regions (indicating a zone of dispersion forces in action) between the extended acene groups.
| Open | Closed |
|---|---|
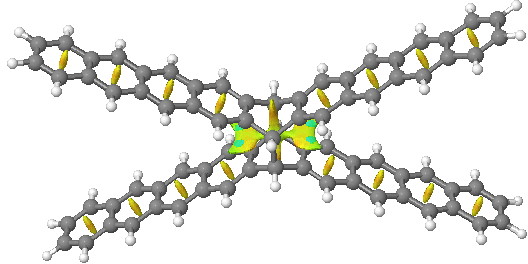 Click for 3D. Open acene |
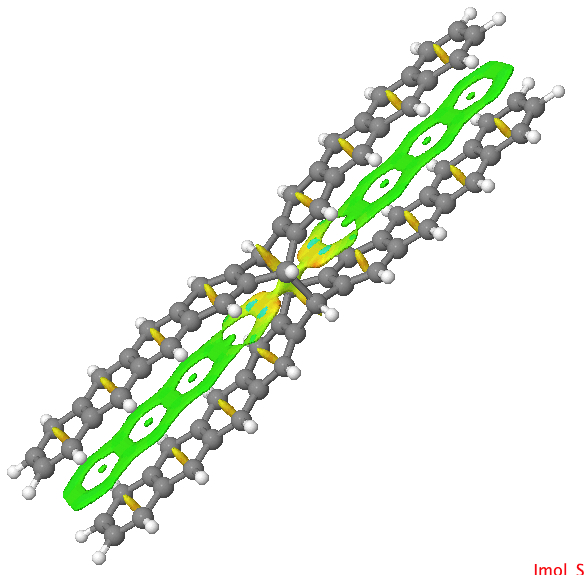 Click for 3D. Closed acene with more green regions. |
Whilst the acene system is an extreme example of this sort of effect, it may well be that even quite small molecules such as benzyl magnesium bromide etherate might manifest effects due to dispersion. After all, 0.3Å is not a particularly small change in geometry! I conclude by noting that isopropyl groups are rather better in their attraction to a phenyl ring than ethyl groups, and so one might speculate whether e.g. the di-isopropyl etherate of benzyl magnesium bromide might be worth someone making?
References
- M.A. Nesbit, D.L. Gray, and G.S. Girolami, "Di-μ-bromido-bis[benzyl(diethyl ether)magnesium]", Acta Crystallographica Section E Structure Reports Online, vol. 68, pp. m942-m942, 2012. https://doi.org/10.1107/s1600536812025445
- S. Ehrlich, H.F. Bettinger, and S. Grimme, "Dispersion‐Driven Conformational Isomerism in σ‐Bonded Dimers of Larger Acenes", Angewandte Chemie International Edition, vol. 52, pp. 10892-10895, 2013. https://doi.org/10.1002/anie.201304674
Additional details
Description
In the previous post I mentioned in passing the Grignard reagent benzyl magnesium bromide as having tetrahedral coordination at Mg. But I have now noticed, largely through spotting Steve Bachrach's post on "Acene dimers – open or closed?" another geometric effect perhaps worthy of note, certainly one not always noted in the past;
Identifiers
- UUID
- fd4fb037-dec6-4098-8c7a-150b7d4b7e98
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=11597
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=11597
Dates
- Issued
-
2013-11-10T14:19:45
- Updated
-
2014-07-19T18:06:33
References
- Nesbit, M. A., Gray, D. L., & Girolami, G. S. (2012). Di-μ-bromido-bis[benzyl(diethyl ether)magnesium]. Acta Crystallographica Section E Structure Reports Online, 68(7), m942–m942. https://doi.org/10.1107/s1600536812025445
- Ehrlich, S., Bettinger, H. F., & Grimme, S. (2013). Dispersion‐Driven Conformational Isomerism in σ‐Bonded Dimers of Larger Acenes. Angewandte Chemie International Edition, 52(41), 10892–10895. https://doi.org/10.1002/anie.201304674
