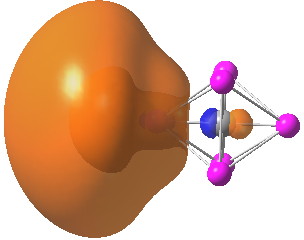
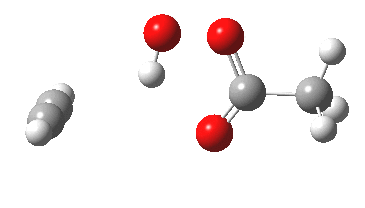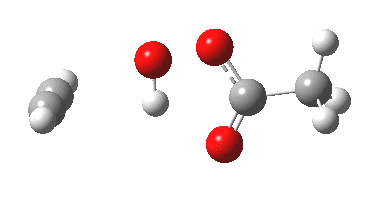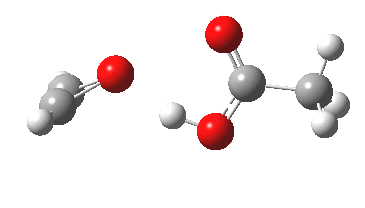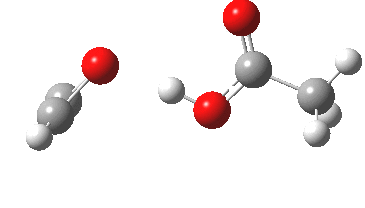
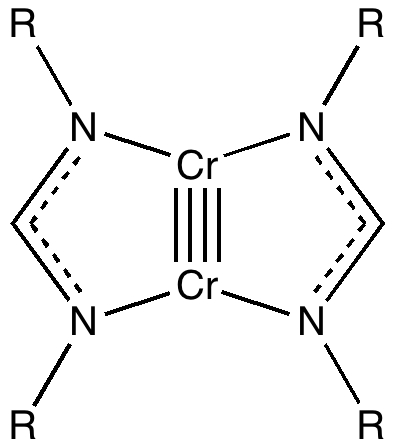
Conferences can be intense, and this one is no exception. After five days, saturation is in danger of setting in. But before it does, I include two more (very) brief things I have learnt. Sason Shaik introduced a theme he first investigated years ago, but for which no experiment had been devised for verification. He revived his theme when a journalist contacted him last year to report exactly such an observation, which I now recount.