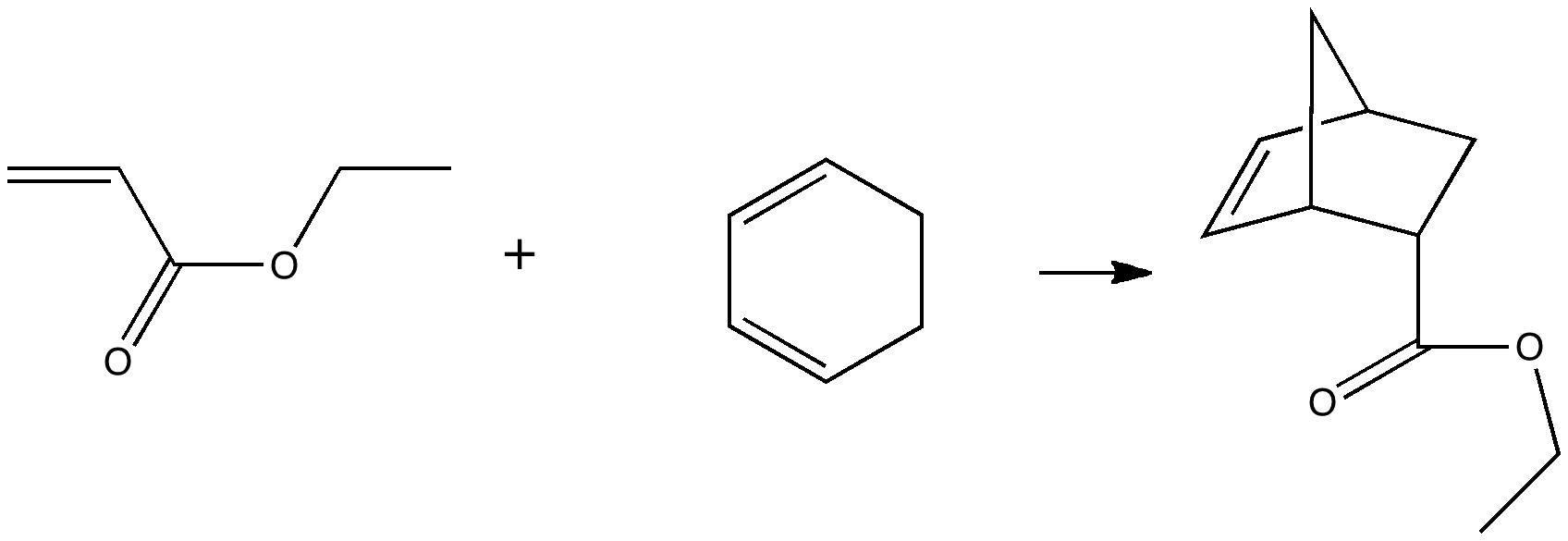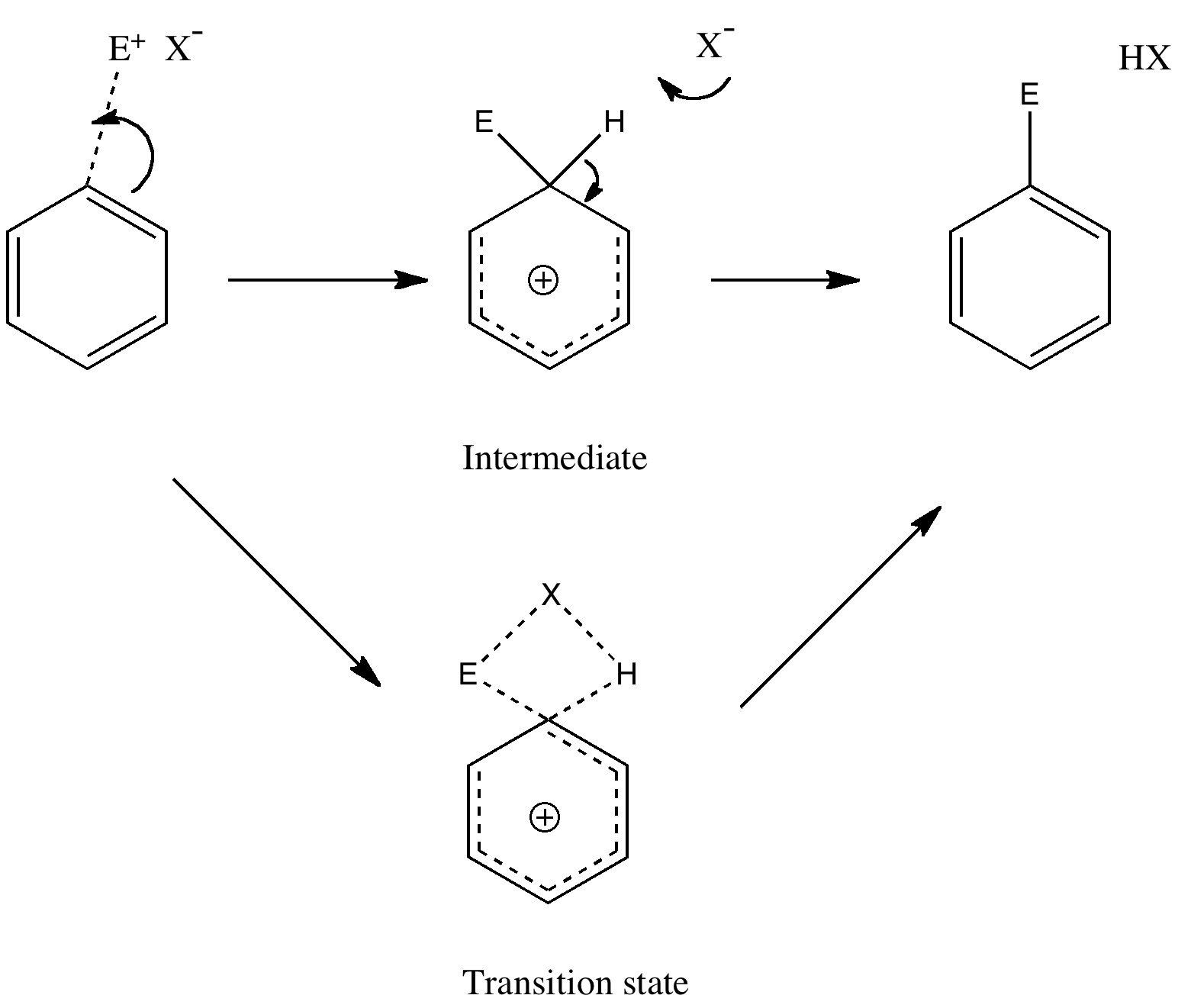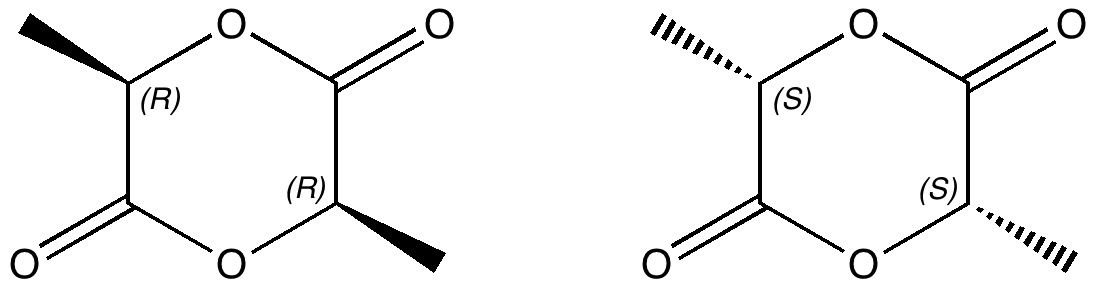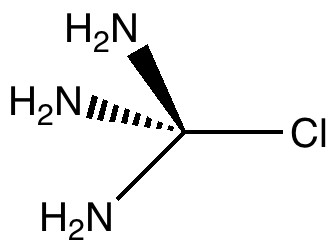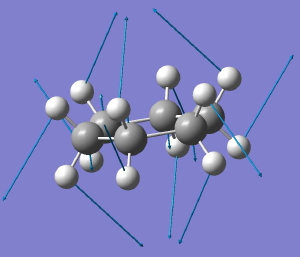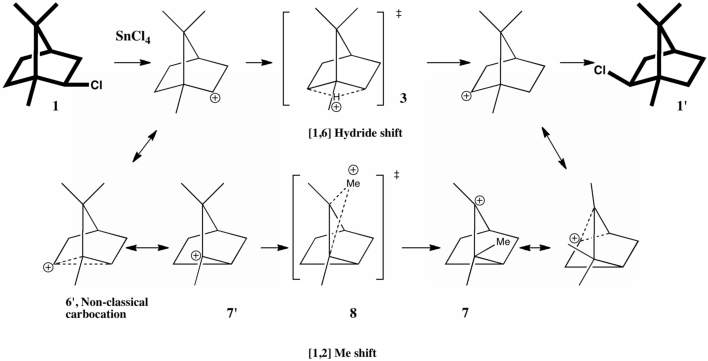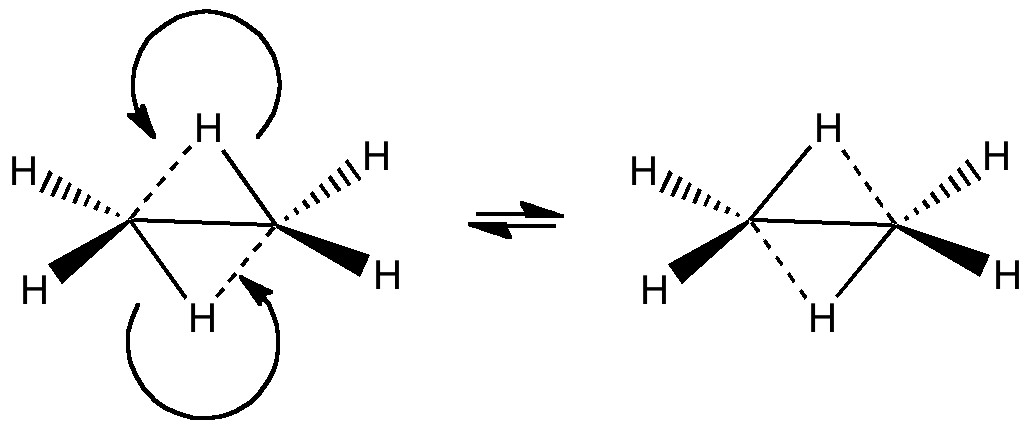
In a time when large (molecules) are considered beautiful (or the corollary that beauty must be big), it is good to reflect that small molecules may teach us something as well. Take ethane. Is there anything left which has not been said about it already? Well, consider the reaction below, in which two hydrogen atoms mutually hop from one carbon to the other. The dyotropic reaction of ethane.