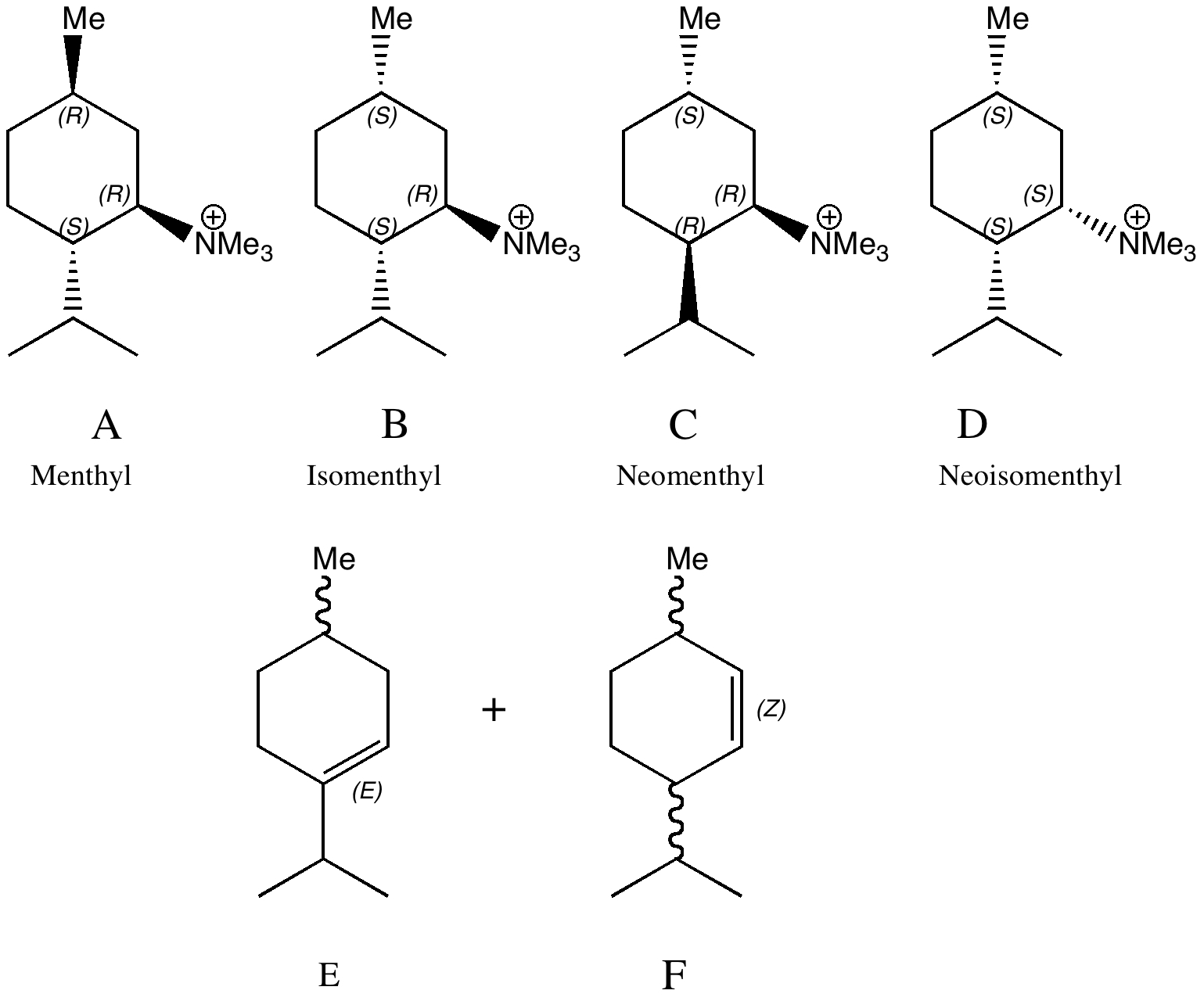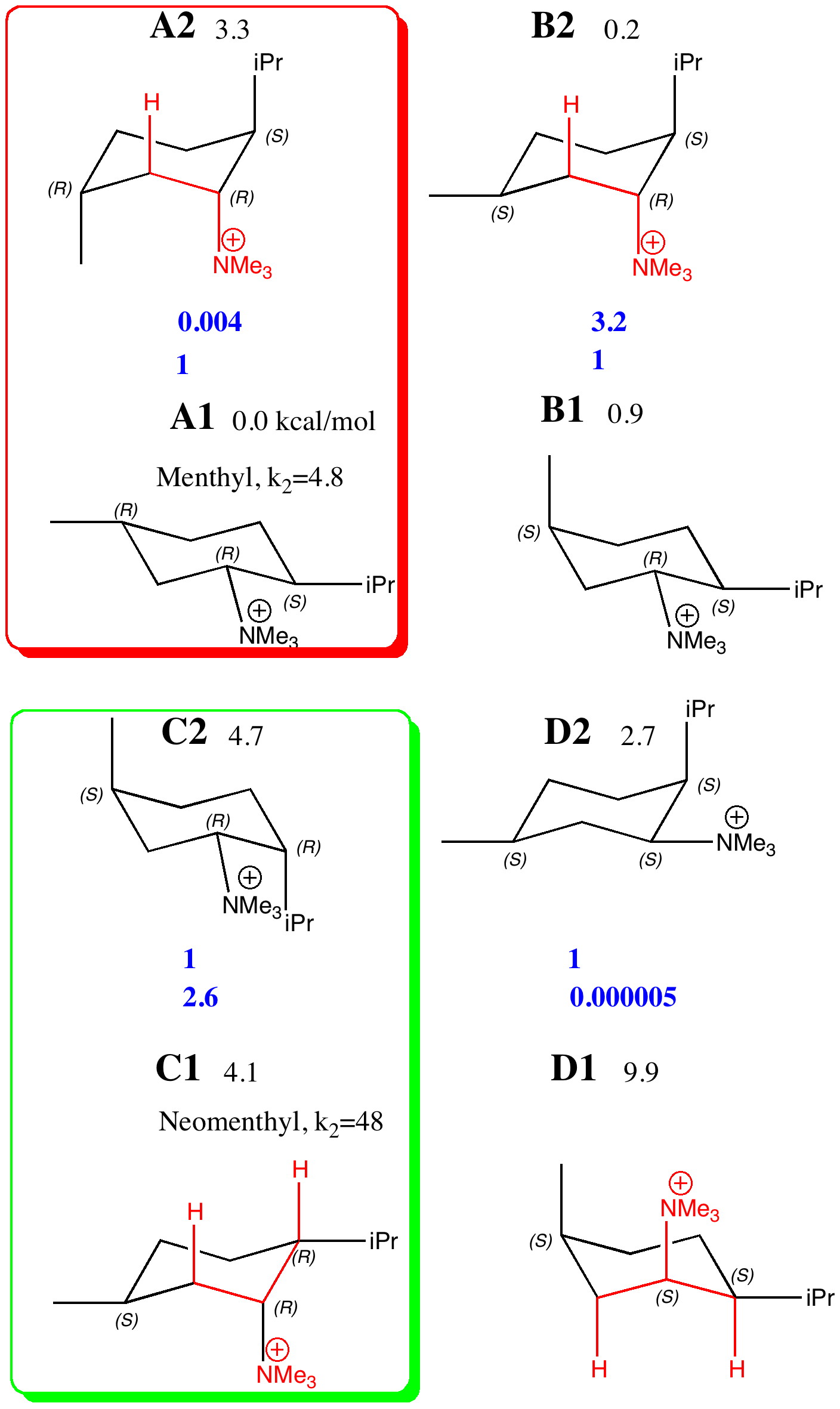
The previous post set out a problem in conformational analysis. Here is my take, which includes an NCI (non-covalent interaction) display as discussed in another post. The lowest energies of the four diastereomers A-D , each in two conformations ( 1/2 ) were calculated at the ωB97D/6-311G(d,p)/SCRF=ethanol level, and are shown here relative to A1 (kcal/mol) as free energies.