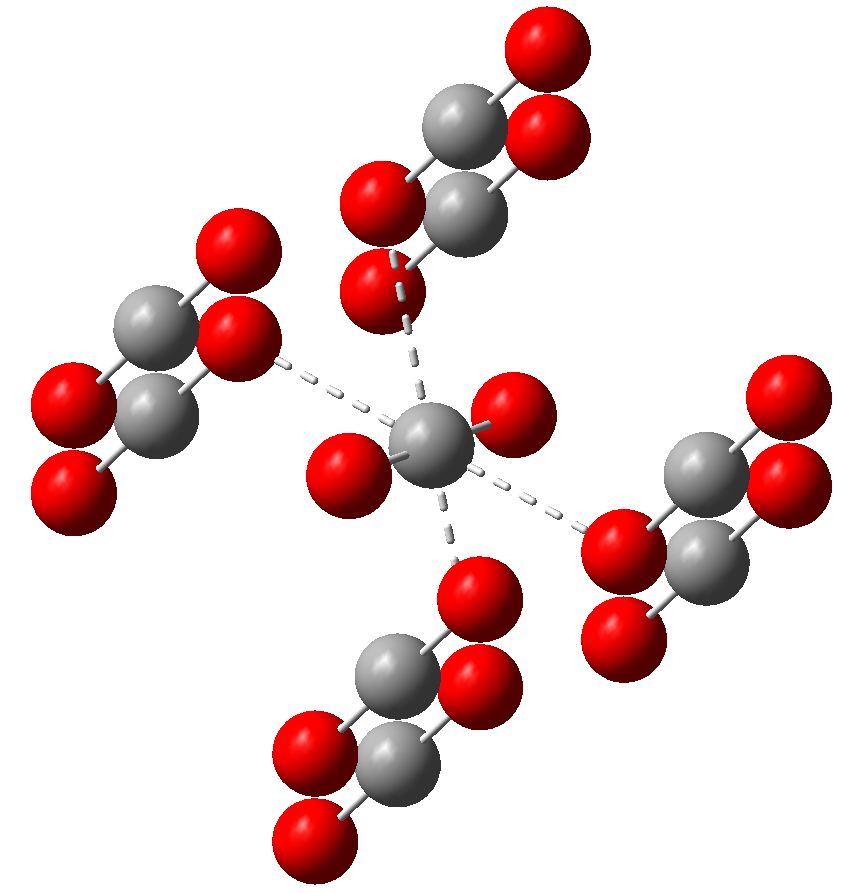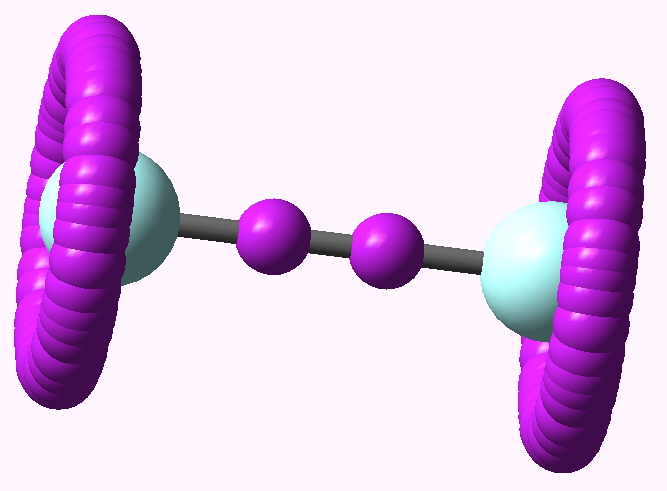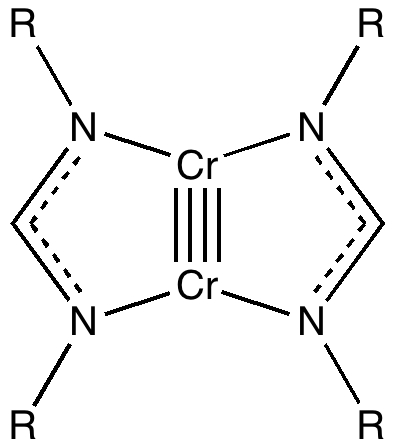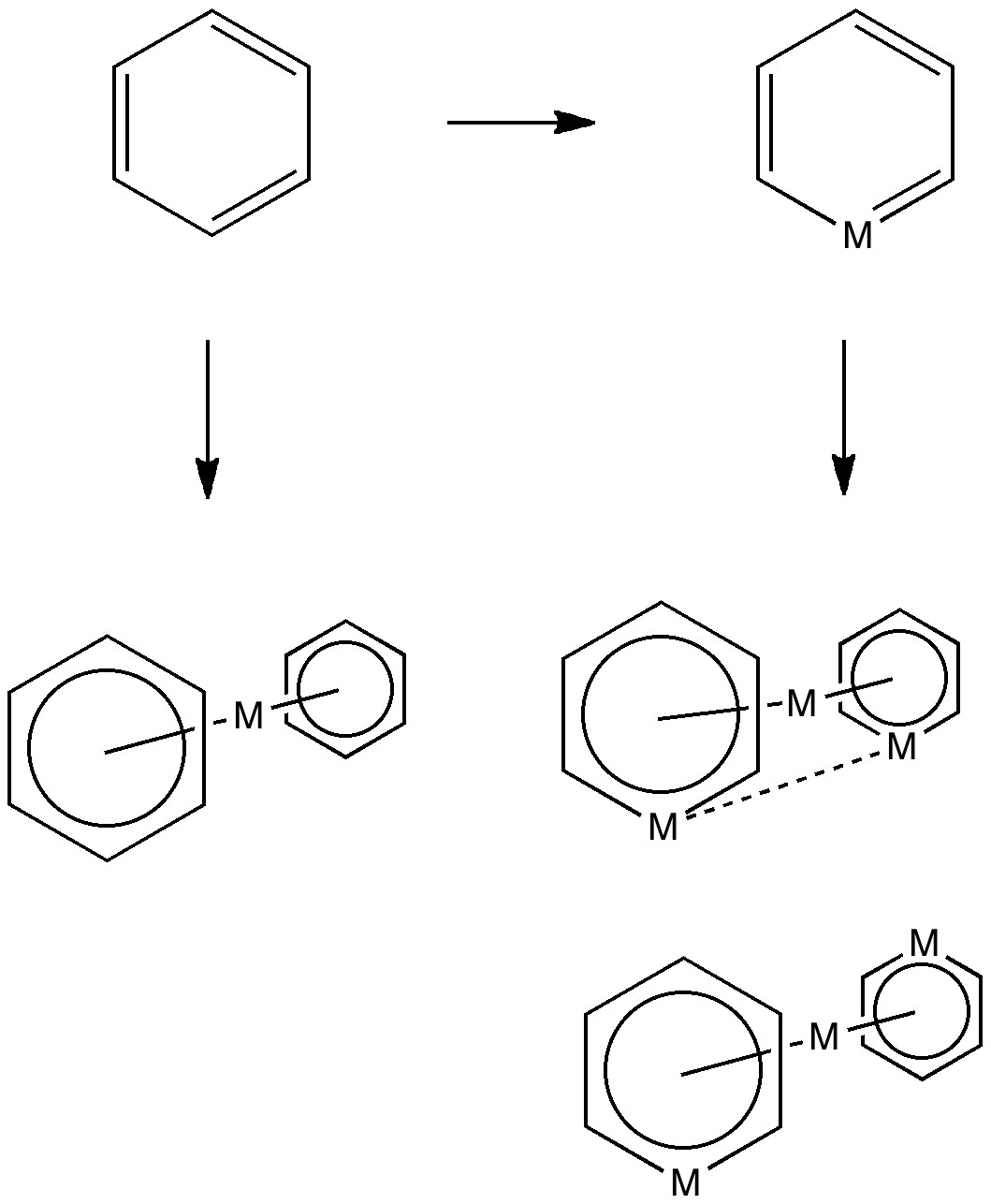
Much like chocolate, some of us metallaholics cannot get enough. So WUQXIP proved an irresistible frolic (DOI: 10.1021/om020789h). Let us start with benzene. It can have metals added in two ways, whilst preserving its essential aromaticity. Triple metal delight. Making a metal sandwich is of course very well known, ferrocene being the first example where the bonding was identified.