My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule.
Rogue Scholar Beiträge
As the title hints, I have been here before. The S N 1 solvolysis mechanism of t-butyl chloride was central to the flourishing of physical organic chemistry from the 1920s onwards, and it appears early on in most introductory lecture courses and text books. There we teach that it is a two-stage mechanism.
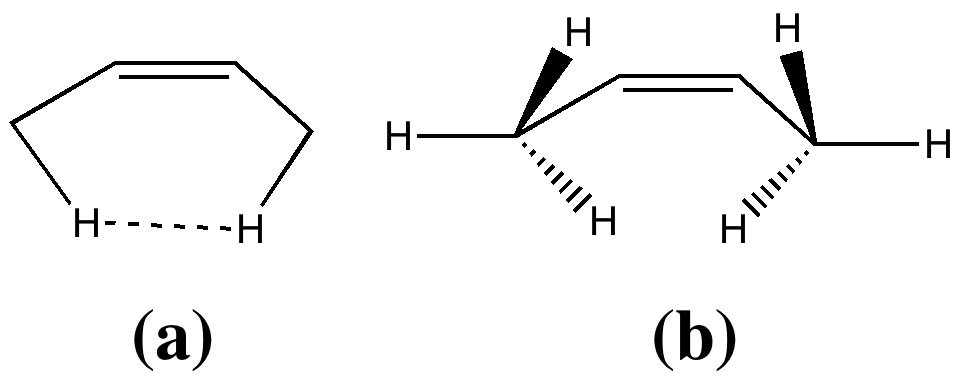
In two previous posts, I have looked at why cis -butene adopts conformation (a) rather than (b). I suggested it boiled down to electronic interactions between the methyl groups and the central alkene resulting in the formation of a H…H “ topological ” bond, rather than attraction between the H…H region to form a weak chemical “ bond “. Here I take a look at what happens when that central C=C bond is gradually removed.
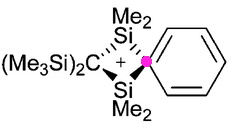
Charges in chemistry, like the grin on Lewis Carroll’s cat, can be mysterious creatures. Take for example the following structure, reported by Paul Lickiss and co-workers (DOI: 10.1039/b513203g). A silenium cation.
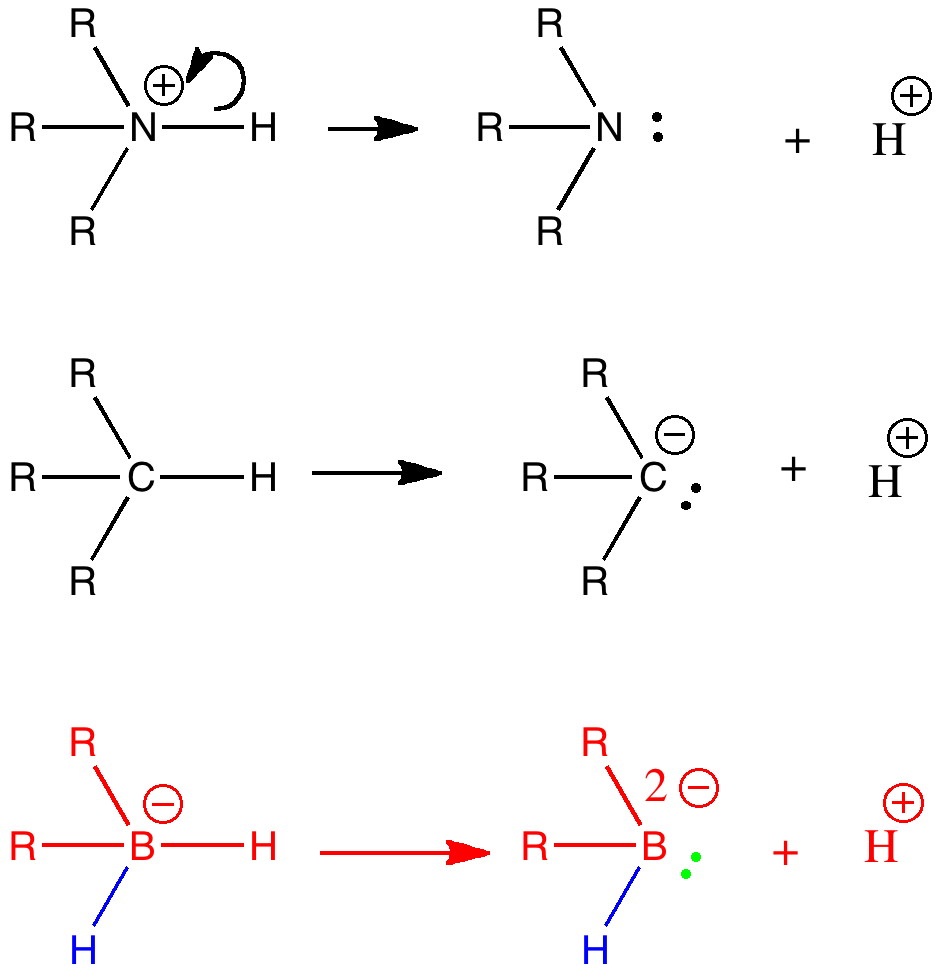
I have often heard the question posed “ how much of chemistry has been discovered? ” Another might be “ has most of chemistry, like low-hanging fruit, already been picked? “. Well, time and time again, one comes across examples which are only a simple diagram or so away from what might be found in any introductory chemistry text, and which would tend to indicate the answers to these questions is a
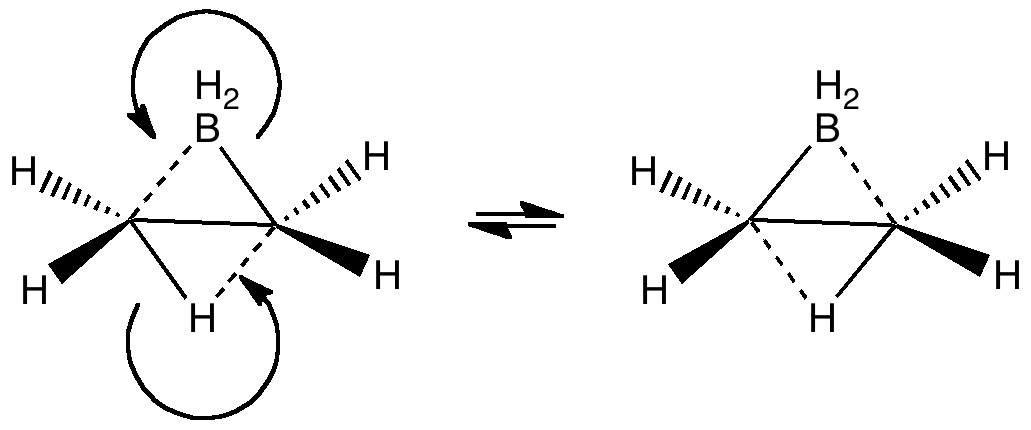
The last two posts have played a game of find the electrons. We saw how the dyotropic rearrangement of ethane borrowed electrons from the C-C bond, and how 1,2,dibromoethane went ionic on us. How about this mixed system, in which a hydrogen and a BH 2 swap their positions? Dyotropic rearrangement involving boron and hydrogen. It is yet again different.

In the previous post, I discussed what we could learn from ethane by forcing it into a pericyclic dyotropic rearrangement. We saw how it voraciously scavenged two electrons from the C-C bond to achieve this. What if we give it more electrons? Thus 1,2-dibromoethane undergoing the same reaction. Dyotropic rearrangement of 1,2-dibromoethane.
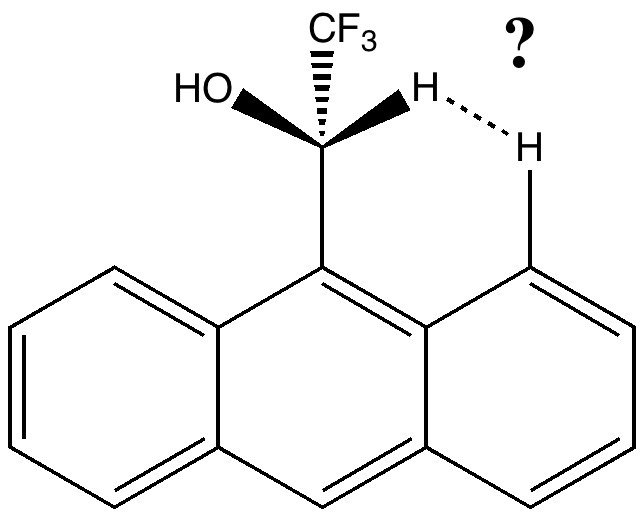
This molecule is not leaving me in peace. It and I first met in 1990 (DO: 10.1039/C39910000765), when we spotted the two unusual π-facial bonds formed when it forms a loose dimer.
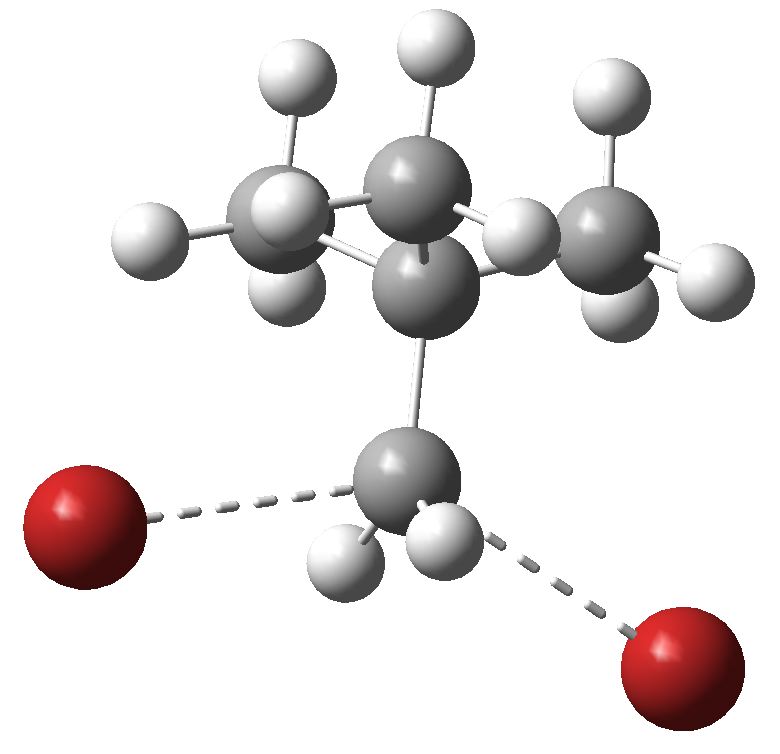
Introductory organic chemistry invariably features the mechanism of haloalkane solvolysis, and introduces both the Sn1 two-step mechanism, and the Sn2 one step mechanism to students. They are taught to balance electronic effects (the stabilization of carbocations) against steric effects in order to predict which mechanism prevails.
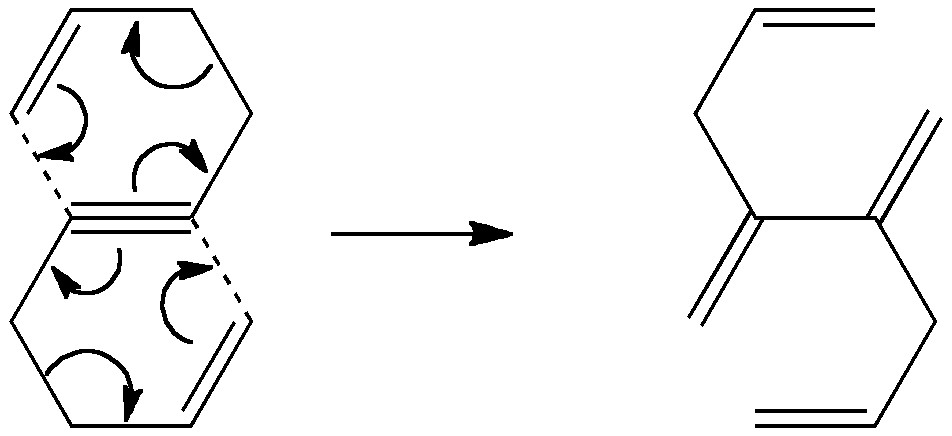
Is there a preferred pack size for electrons on the move? Or put less flamboyantly, is there an optimum, and a maximum number of arrows (electron pairs) that one might push in revealing the mechanism of a concerted reaction? A sort of village-instinct for electrons.