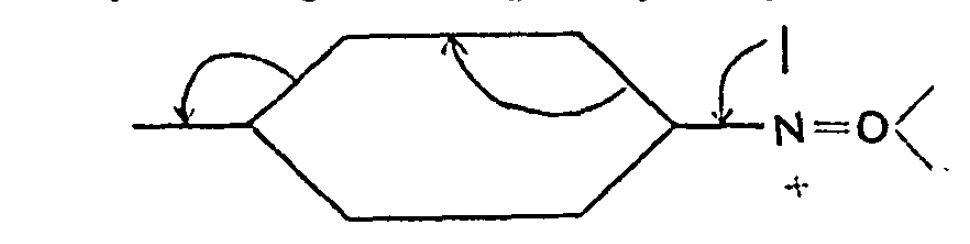
Chemists now use the term “curly arrows” as a language to describe the electronic rearrangements that occur when a (predominately organic) molecule transforms to another – the so called chemical reaction. It is also used to infer, via valence bond or resonance theory, what the mechanistic implications of that reaction are.