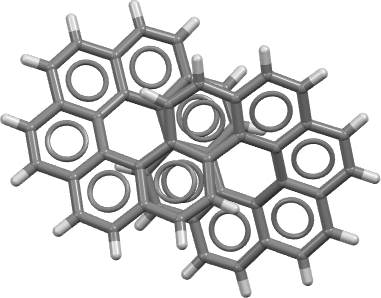
The annual “molecule of the year” results for 2021 are now available … and the winner is Infinitene .[cite]10.33774/chemrxiv-2021-pcwcc[/cite],[cite]10.1021/jacs.1c10807[/cite] This is a benzocirculene in the form of a figure eight loop (the infinity symbol), a shape which is also called a lemniscate [cite]10.1021/jo801022b[/cite] after the mathematical (2D) function due to Bernoulli.