Nature has produced most natural molecules as chiral objects, which means the molecule can come in two enantiomeric forms, each being the mirror image of the other. When a natural product is synthesised in a laboratory, a chiral synthesis means just one form is made, and then is compared with the natural product to see if it matches.
Messaggi di Rogue Scholar
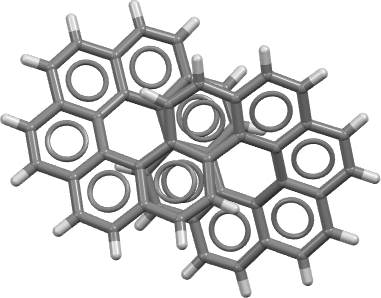
The annual “molecule of the year” results for 2021 are now available … and the winner is Infinitene .[cite]10.33774/chemrxiv-2021-pcwcc[/cite],[cite]10.1021/jacs.1c10807[/cite] This is a benzocirculene in the form of a figure eight loop (the infinity symbol), a shape which is also called a lemniscate [cite]10.1021/jo801022b[/cite] after the mathematical (2D) function due to Bernoulli.
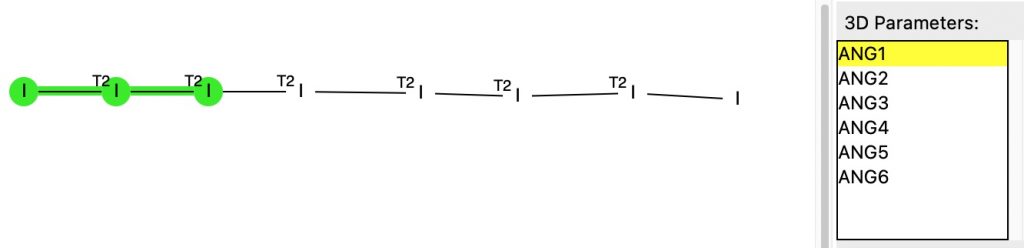
The previous post described the fascinating 170-year history of a crystalline compound known as Herapathite and its connection to the mechanism of the Finkelstein reaction via the complex of Na + I 2 – (or Na 2 2+ I 4 2- ). Both compounds exhibit (approximately) linear chains of iodine atoms in their crystal structures, a connection which was discovered serendipitously.
On October 13, 2021, the historical group of the Royal Society of Chemistry organised a symposium celebrating ~150 years of the history of (molecular) chirality. We met for the first time in person for more than 18 months and were treated to a splendid and diverse program about the subject. The first speaker was Professor John Steeds from Bristol, talking about the early history of light and the discovery of its polarisation.
My momentum of describing early attempts to use optical rotation to correlate absolute configuration of small molecules such as glyceraldehyde and lactic acid with their optical rotations has carried me to L-Malic acid (below labelled as ( S )-Malic acid). The measured optical rotatory dispersion curve at low wavelengths is shown below (dashed line for Malic acid, solid line for Lactic acid). A sign inversion
I have been discussing some historical aspects of the absolute configuration of molecules and how it was connected to their optical rotations. The nomenclature for certain types of molecules such as sugars and less commonly amino acids includes the notation (+) to indicate that the specific optical rotation of the molecule has a positive (rather than a negative) value.
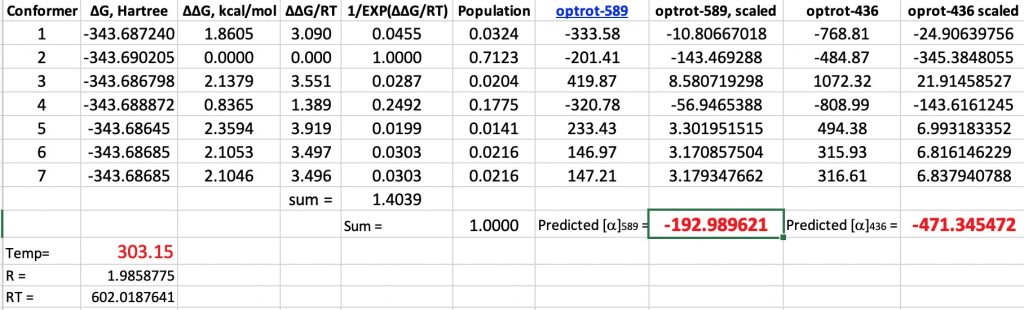
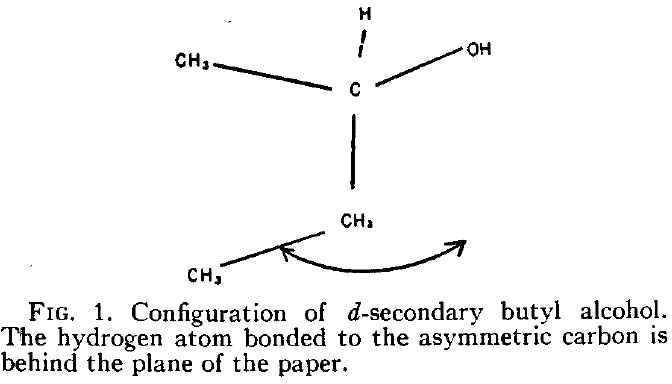
In this series of posts on optical rotations, I firstly noted Kirkwood’s 1937 attempt to correlate the optical rotation of butan-2-ol with its absolute configuration.
Text books often show the following diagram, famously consolidated over many years by Emil Fischer from 1891 onwards. At the top sits D -(+)-glyceraldehyde, to which all the monosaccharides below are connected by painstaking chemical transformations.
Some areas of science progressed via very famous predictions that were subsequently verified by experiments. Think of Einstein and gravitational waves or of Dirac and the positron. There are fewer well-known examples in chemistry; perhaps Watson and Crick’s prediction of the structure of DNA, albeit based on the interpretation of an existing experimental result.
I mentioned in my last post an unjustly neglected paper from that golden age of 1951-1953 by Kirkwood and co. They had shown that Fischer’s famous guess for the absolute configurations of organic chiral molecules was correct. The two molecules used to infer this are shown below.