A game chemists often play is to guess the mechanism for any given reaction. I thought I would give it a go for the decomposition of the tris-peroxide shown below.
Messaggi di Rogue Scholar
The mechanism of forming an oxime from nucleophilic addition of a hydroxylamine to a ketone is taught early on in most courses of organic chemistry. Here I subject the first step of this reaction to form a tetrahedral intermediate to quantum mechanical scrutiny. The first decision is to decide which atom of the hydroxylamine acts as the nucleophile. Reaction 1 shows the oxygen and reaction 2 the nitrogen.
This reaction looks simple but is deceptively complex.
This is a continuation of the previous post exploring the transition state geometries of various types of ring closure as predicted by Baldwin’s rules. I had dealt with bond formation to a trigonal (sp 2 ) carbon; now I add a digonal (sp) example (see an interesting literature variation).
The Baeyer-Villiger rearrangement was named after its discoverers, who in 1899 described the transformation of menthone into the corresponding lactone using Caro’s acid (peroxysulfuric acid). The mechanism is described in all text books of organic chemistry as involving an alkyl migration.
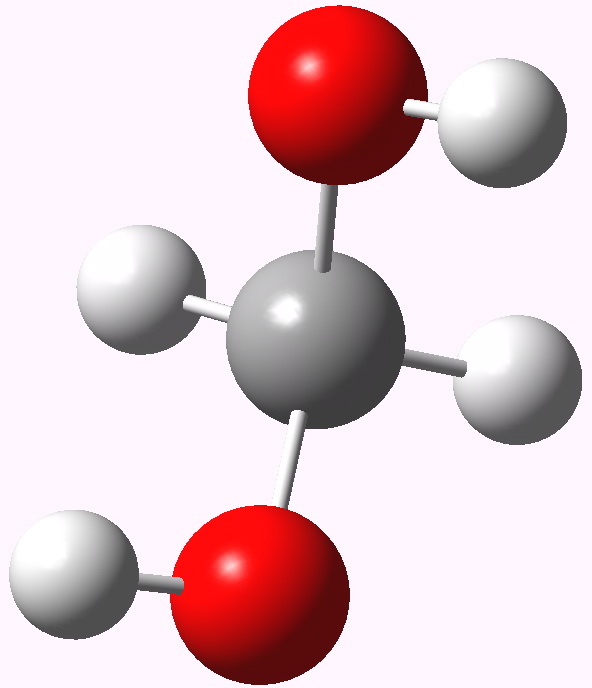
In my previous post I speculated why bis (trifluoromethyl) ketone tends to fully form a hydrate when dissolved in water, but acetone does not. Here I turn to asking why formaldehyde is also 80% converted to methanediol in water? Could it be that again, the diol is somehow preferentially stabilised compared to the carbonyl precursor and if so, why? Methanediol. The lowest energy geometry is shown above.
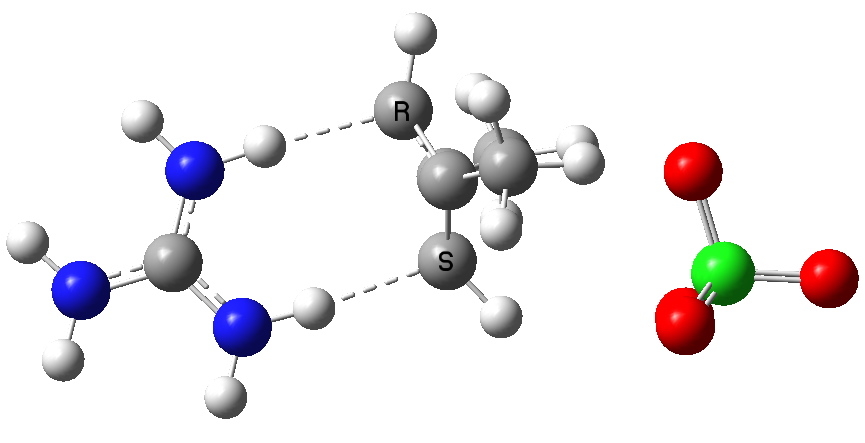
To (mis)quote Oscar Wilde again, ““To lose one methyl group may be regarded as a misfortune; to lose both looks like carelessness.” Here, I refer to the (past) tendency of molecular modellers to simplify molecular structures. Thus in 1977, quantum molecular modelling, even at the semi-empirical level, was beset by lost groups. One of my early efforts (DOI: 10.1021/ja00465a005) was selected for study because it had nothing left to lose;
Organic chemistry has some no-go areas, where few molecules dare venture. One of them is described by a concept known as anti-aromaticity. Whereas aromatic molecules are favoured species, their anti-equivalent is avoided. I previously illustrated this (Hückel rule) with cyclopropenium anion.
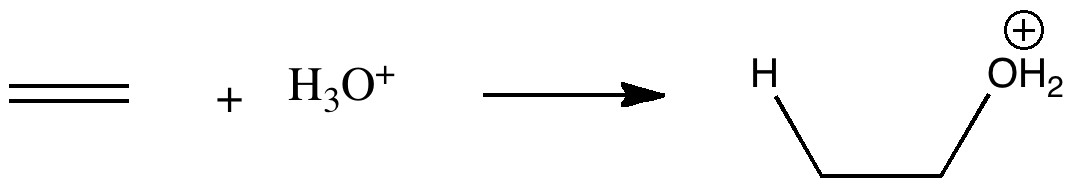
The hydration of an alkene by an acid is one of those fundamental reactions, taught early on in most chemistry courses. What can quantum mechanics teach us about the mechanism of the reaction? The hydration of ethene by a hydronium cation.
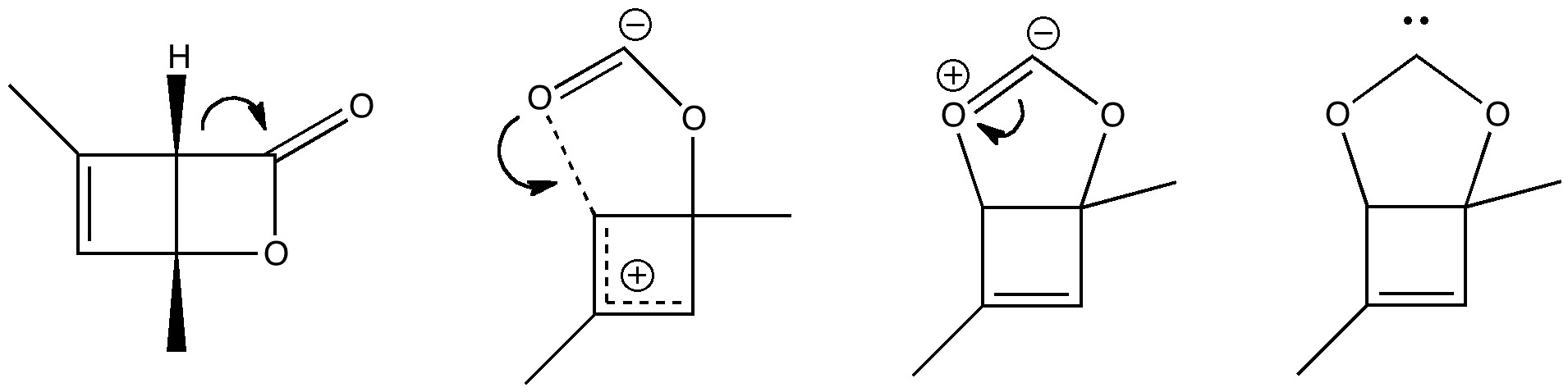
The (hopefully tongue-in-cheek) title Mindless chemistry was given to an article reporting[cite]10.1021/jp057107z[/cite] an automated stochastic search procedure for locating all possible minima with a given composition using high-level quantum mechanical calculations. “Many new structures, often with nonintuitive geometries, were found”. Well, another approach is to follow unexpected hunches.