HCl reacting with a carbonyl compound (say formaldehyde) sounds pretty simple. But often the simpler a thing looks, the more subtle it is under the skin. And this little reaction is actually my prelude to the next post. The mechanism is studied using ωB97XD/6-311G(d,p) with a simulated solvent (acetic acid) included (but not explicit solvent setting up any hydrogen bonds). Transition state HCl + H2C=O. Click for 3D animation.
Messaggi di Rogue Scholar
The mechanism of the reaction of alkenes known as ozonolysis was first set out in its modern form by Criegee. The crucial steps, (a), (b) and (d), are all pericyclic cycloaddition/eliminations.
During the 1960s, a holy grail of synthetic chemists was to devise an efficient route to steroids. R. B. Woodward was one the chemists who undertook this challenge, starting from compounds known as dienones ( e.g. 1 ) and their mysterious conversion to phenols ( e.g. 2 or 3 ) under acidic conditions.
Astronomers who discover an asteroid get to name it, mathematicians have theorems named after them. Synthetic chemists get to name molecules (Hector’s base and Meldrum’s acid spring to mind) and reactions between them. What do computational chemists get to name? Transition states! One of the most famous of recent years is the Houk-List.
Chemists love a mystery as much as anyone. And gaps in patterns can be mysterious. Mendeleev’s period table had famous gaps which led to new discovery. And so from the 1890s onwards, chemists searched for the perbromate anion, BrO 4 – . It represented a gap between perchlorate and periodate, both of which had long been known. As the failure to turn up perbromate persisted, the riddle deepened.
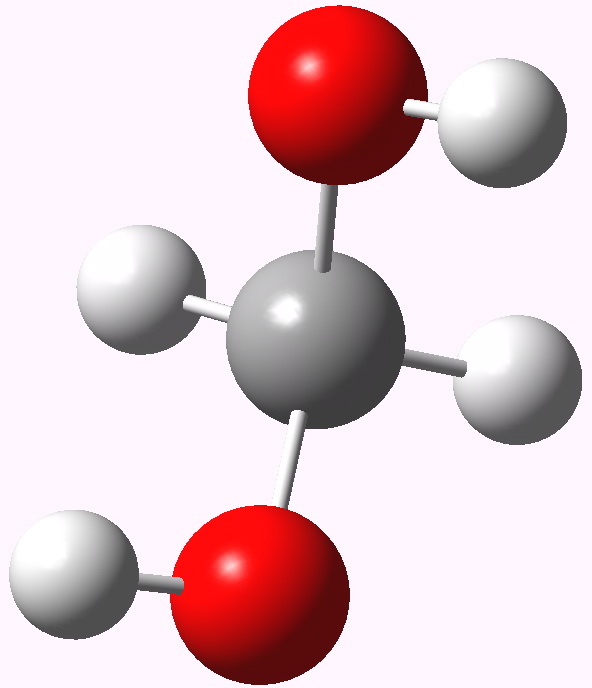
In my previous post I speculated why bis (trifluoromethyl) ketone tends to fully form a hydrate when dissolved in water, but acetone does not. Here I turn to asking why formaldehyde is also 80% converted to methanediol in water? Could it be that again, the diol is somehow preferentially stabilised compared to the carbonyl precursor and if so, why? Methanediol. The lowest energy geometry is shown above.
The equilibrium for the hydration of a ketone to form a gem-diol hydrate is known to be highly sensitive to substituents. Consider the two equilibria: For propanone, it lies almost entirely on the left, whereas for the hexafluoro derivative it is almost entirely on the right. The standard answer to this is that electron-withdrawing substituents destabilize the carbonyl compound more than the hydrate.
The so-called E2 elimination mechanism is another one of those mainstays of organic chemistry. It is important because it introduces the principle that anti-periplanarity of the reacting atoms is favoured over other orientations such as the syn-periplanar form; Barton used this principle to great effect in developing the theory of conformational analysis. Here I explore its origins.
Chemistry rarely makes it to the cover of popular science magazines. Thus when this week, the New Scientist ran the headline “ Forbidden chemistry. Reactions they said could never happen ”, I was naturally intrigued. The examples included Woodward and Hoffmann’s “ symmetry-forbidden ” reactions, which have been the subject of several posts here already.
The tetrahedral intermediate is one of those iconic species on which the foundation of reaction mechanism in organic chemistry is built. It refers to a (normally undetected and hence merely inferred) species formed initially when a nucleophilic reagent attacks a carbonyl compound. Its importance to understanding the activity of enzymes cannot be overstated.