I have written a few times about the so-called “anomeric effect”, which relates to stereoelectronic interactions in molecules such as sugars bearing a tetrahedral carbon atom with at least two oxygen substituents. The effect can be detected when the two C-O bond lengths in such molecules are inspected, most obviously when one of these bonds has a very different length from the other.
Rogue Scholar Posts
First, a very brief history of scholarly publishing, starting in 1665[1] when scientific journals started to be published by learned societies. This model continued until the 1950s, when commercial publishers such as Pergamon Press started with their USP (unique selling point) of rapid time to publication of ~3 months,[2] compared to typical times for many learned society publishers of 2 years or longer.
In an earlier post on this topic,[1] ‡ I described how the curly-arrows describing the mechanism of a nucleophilic addition at a carbonyl group choreograph in two distinct ways, as seen in red or blue below. The arrows in red can be described as firstly addition to the carbonyl group to form either a transient intermediate (a two-step process) or instead a formal transition state state as a concerted single-step mechanism.

Respiratory pigments are metalloproteins that transport O 2 , the best known being the bright red/crimson coloured hemoglobin in human blood. The colour derives from Fe 2+ at the core of a tetraporphyrin ring. But less well known is blue blood , and here the colour derives from an oxyhemocyanin unit based on Cu 1+ (the de-oxy form is colourless) rather than iron.
One of the important aspects of chemical reaction mechanisms is the order in which things happen. More specifically, the order in which bonds make or break when there are more than two involved in undertaking a reaction.
What’s a Journal For? This debate has been raging ever since preprint servers were introduced as far back as 1991!
Sometimes you come across a reaction which is so simple in concept that you wonder why it took so long to be accomplished in practice.
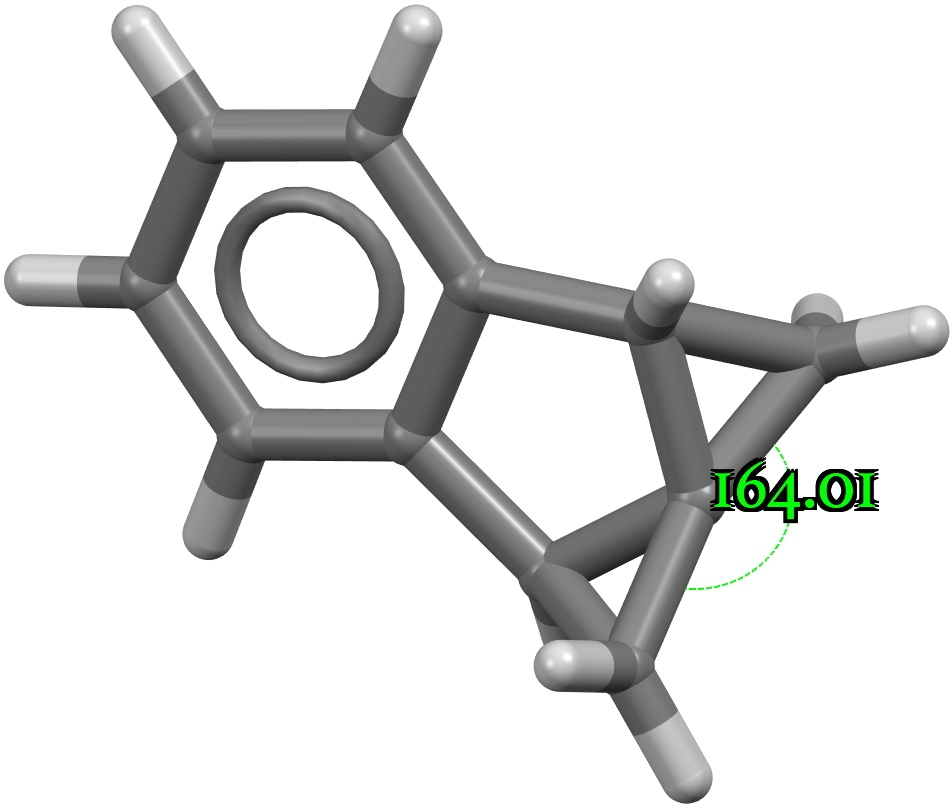
Four-coordinate carbon normally adopts a tetrahedral shape, where the four angles at the carbon are all 109.47°. But how large can that angle get, and can it even get to be 180°? A search of the CSD (crystal structure database) reveals a spiropentane as having the largest such angle, VAJHAP with 164°[1] Because crystal structures might have artefacts such as disorder etc, it is always good to check this with a calculation;
Previously, a mechanism with a reasonable predicted energy was modelled for the isomerisation of an oxetane carboxylic acid to a lactone by using two further molecules of acid to transfer the proton and in the process encouraging an Sn2 reaction with inversion to open the oxetane ring. We are now ready to explore variations to this mechanism to see what happens.
I have long been fascinated by polymers of either carbon dioxide, † or carbon monoxide, or combinations of both. One such molecule, referred to as dioxane tetraketone when it was featured on the ACS molecule-of-the-week site and also known as the anhydride of oxalic acid, or more formally 1,4-dioxane-2,3,5,6-tetraone, has been speculated upon for more than a century.[1] The history of chemistry has many molecules whose