The previous post described how the acid catalysed ring opening of propene epoxide by an alcohol (methanol) is preceded by pre-protonation of the epoxide oxygen to form a “hidden intermediate” on the concerted intrinsic reaction pathway to ring opening.
Rogue Scholar Posts
I mentioned in the last post that one can try to predict the outcome of electrophilic aromatic substitution by approximating the properties of the transition state from those of either the reactant or the (presumed Wheland) intermediate by invoking Hammond’s postulate[1]. A third option is readily available nowadays; calculate the transition state directly. Here are the results of exploring this third variation.
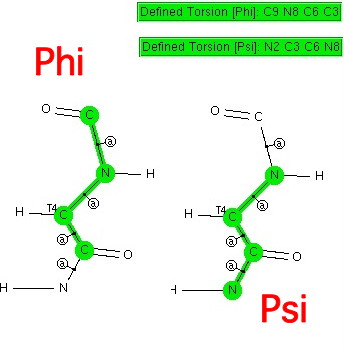
This is really just a postscript to the previous post. There I showed how a search of the (small molecule) crystal database revealed the s-cis conformation about the N-C amide bond (the one with partial double bond character that prevents rotation) and how this conformation means that a C-H approaches quite closely to an adjacent oxygen. It is a tiny step from that search to a related, and very famous one named after Ramachandran[1].
In a previous post, I set out how to show how one can reduce a 1 H NMR spectrum to the structure [A] below. I speculated how a further test could be applied to this structure; back predicting its spectrum using just quantum mechanics. Overkill I know, but how well might the two match? The process must start by considering the conformational possibilities of [A]. Each will have a different predicted spectrum.
It is always rewarding when one comes across a problem in chemistry that can be solved using a continuous stream of rules and logical inferences from them. The example below[1] is one I have been using as a tutor in organic chemistry for a few years now, and I share it here. It takes around 50 minutes to unravel with students.
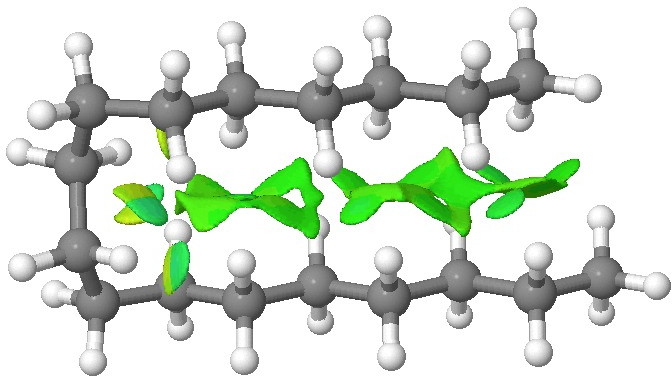
We tend to think of simple hydrocarbons as relatively inert and un-interesting molecules. However, a recent article[1], which was in fact highlighted by Steve Bachrach on his blog , asks what “ The Last Globally Stable Extended Alkane ” might be. In other words, at what stage does a straight-chain hydrocarbon fold back upon itself, and no significant population of the linear form remain?
This is an interesting result I got when studying the [1,4] sigmatropic rearrangement of heptamethylbicyclo-[3.1.0]hexenyl cations. It fits into the last lecture of a series on pericyclic mechanisms, and just before the first lecture on conformational analysis. This is how they join. The experiment it relates to[1] may well be a contender for the top ten list of most influential experiments ever conducted in chemistry.
I noted briefly in discussing why Birch reduction of benzene gives 1,4-cyclohexadiene (diagram below) that the geometry of the end-stage pentadienyl anion was distorted in the presence of the sodium cation to favour this product. This distortion actually has some pedagogic value, and so I elaborate this here.
The Birch reduction is a classic method for partially reducing e.g. aryl ethers using electrons (from sodium dissolved in ammonia) as the reductant rather than e.g. dihydrogen. As happens occasionally in chemistry, a long debate broke out over the two alternative mechanisms labelled O (for ortho protonation of the initial radical anion intermediate) or M (for meta protonation).
Not a few posts on this blog dissect the mechanisms of well known text-book reactions. But one reaction type where there are few examples on these pages are reductions. These come in three types; using electrons, using a hydride anion and using di-hydrogen. Here I first take a closer look at the third type, and in particular di-hydrogen as delivered from di-imide.