Since I have gotten into the habit of quoting some of my posts in other contexts, I have started to also archive them using WebCite. One can quote the resulting archive as: Rzepa, Henry. Quintuple bonds. 2010-04-18. URL:http://www.ch.ic.ac.uk/rzepa/blog/?p=1722. Accessed: 2010-04-18. (Archived by WebCite ® at http://www.webcitation.org/5p5BtuzSH) There is one issue though.
Rogue Scholar Posts
What constitutes an “impossible molecule”? Well, here are two, the first being the topic of a recent article[1]. The second is a favourite of organic chemistry tutors, to see if their students recognise it as an unusual (= impossible) form of a much better known molecule. Perhaps we could define impossible molecules into two slightly different classes.
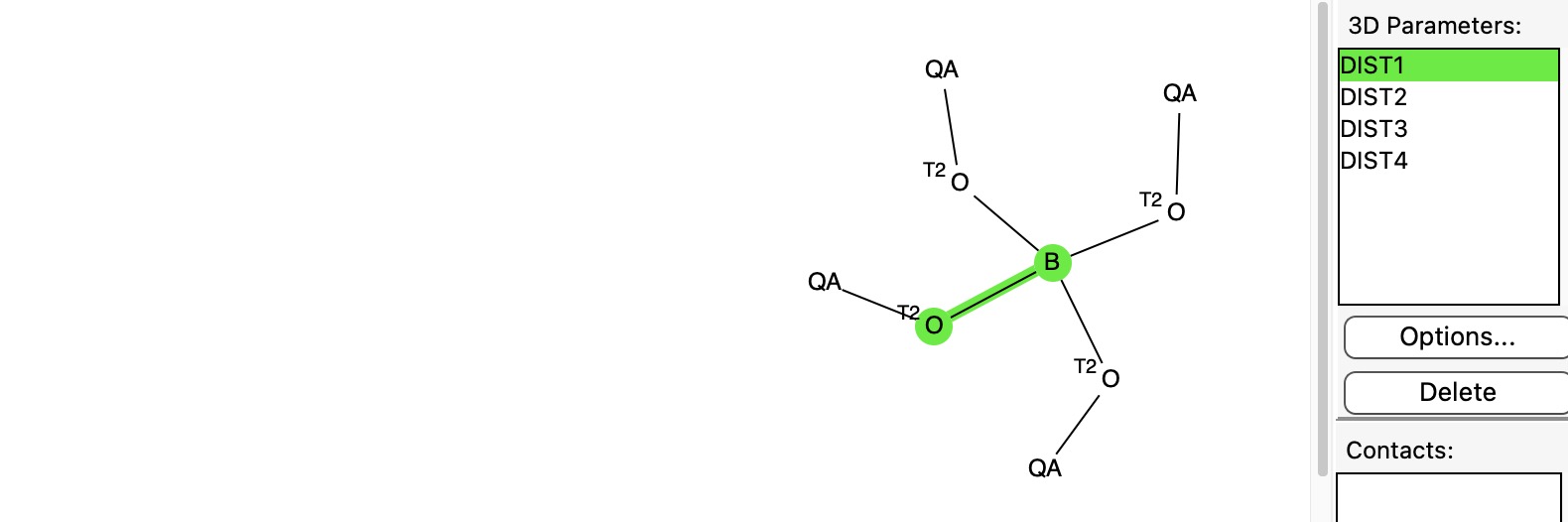
In an earlier post, I discussed[1] a phenomenon known as the “anomeric effect” exhibited by tetrahedral carbon compounds with four C-O bonds. Each oxygen itself bears two bonds and has two lone pairs, and either of these can align with one of three other C-O bonds to generate an anomeric effect. Here I change the central carbon to a boron to explore what happens, as indeed I promised earlier.
In the mid to late 1990s as the Web developed, it was becoming more obvious that one area it would revolutionise was of scholarly journal publishing. Since the days of the very first scientific journals in the 1650s, the medium had been firmly rooted in paper. Even printed colour only became common (and affordable) from the 1980s. An opportunity to move away from these restrictions was provided by the Web.
I have written a few times about the so-called “anomeric effect”, which relates to stereoelectronic interactions in molecules such as sugars bearing a tetrahedral carbon atom with at least two oxygen substituents. The effect can be detected when the two C-O bond lengths in such molecules are inspected, most obviously when one of these bonds has a very different length from the other.
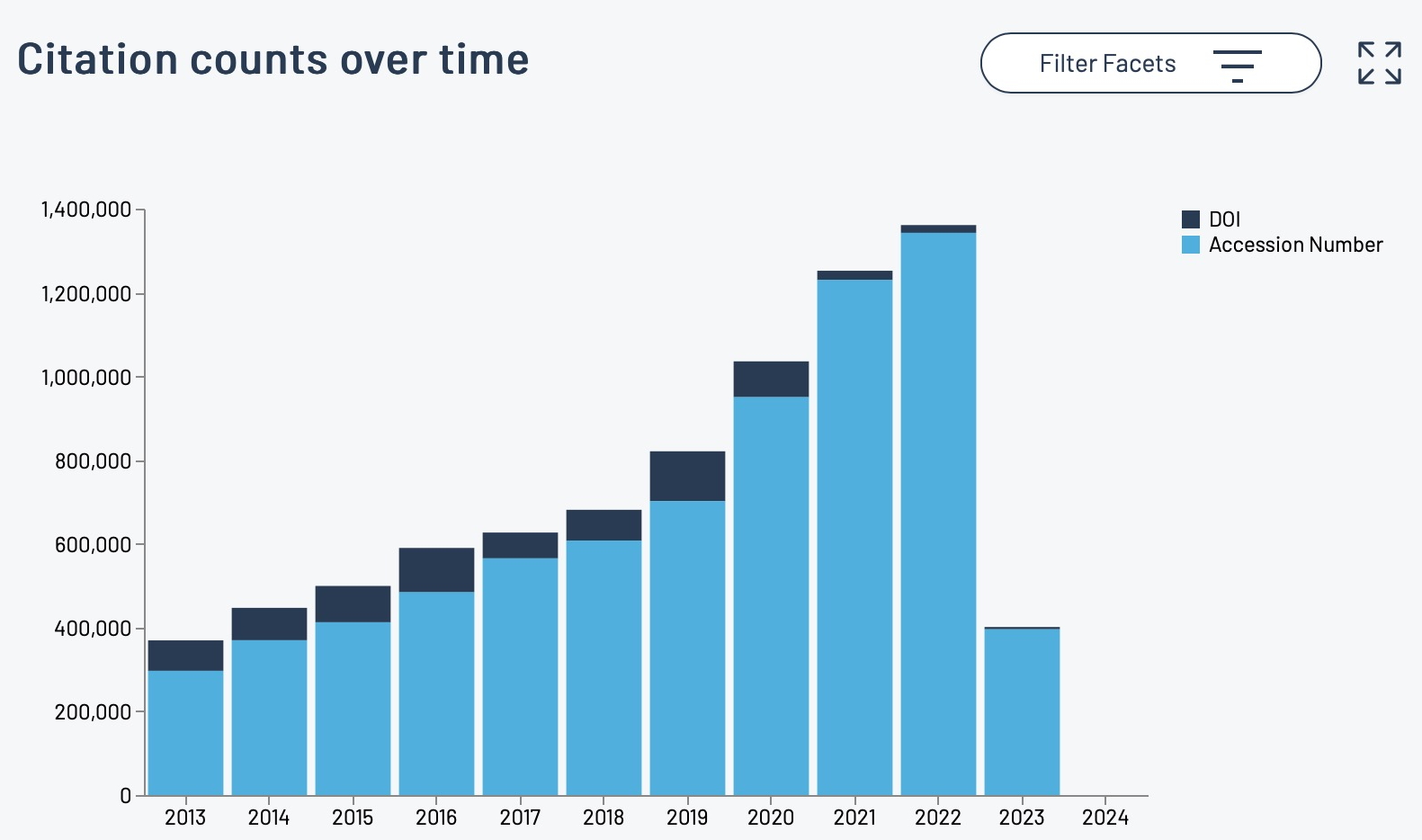
The recent release of the DataCite Data Citation corpus, which has the stated aim of providing “a trusted central aggregate of all data citations to further our understanding of data usage and advance meaningful data metrics” made me want to investigate what the current state of citing data in the area of chemistry might be. Chemistry is known to be a “data rich” science (as most of the physical sciences are) and here on this very blog I
Following on from my template exploration of the Wilkinson hydrogenation catalyst, I now repeat this for the Grubbs variant of the Alkene metathesis reaction. As with the Wilkinson, here I focus on the stereochemistry of the mechanism as first suggested by Chauvin[1], an aspect lacking in eg the Wikipedia entry.
In the late 1980s, as I recollected here[1] the equipment needed for real time molecular visualisation as it became known as was still expensive, requiring custom systems such as Evans and Sutherland PS390 workstations.

On 24th January 1984, the Macintosh computer was released, as all the media are informing us. Apparently, some are still working. I thought I would give my own personal recollections of that period. In fact, the Mac reached UK stores via a dealership only in 1985.
Geoffrey Wilkinson first reported his famous work on the hydrogenation catalyst that now bears his name in 1965[1] and I met him at Imperial College around 1969 and again when I returned there in 1977.