I have written a few times about the so-called “anomeric effect”, which relates to stereoelectronic interactions in molecules such as sugars bearing a tetrahedral carbon atom with at least two oxygen substituents. The effect can be detected when the two C-O bond lengths in such molecules are inspected, most obviously when one of these bonds has a very different length from the other.
Rogue Scholar Posts
First, a very brief history of scholarly publishing, starting in 1665[1] when scientific journals started to be published by learned societies. This model continued until the 1950s, when commercial publishers such as Pergamon Press started with their USP (unique selling point) of rapid time to publication of ~3 months,[2] compared to typical times for many learned society publishers of 2 years or longer.
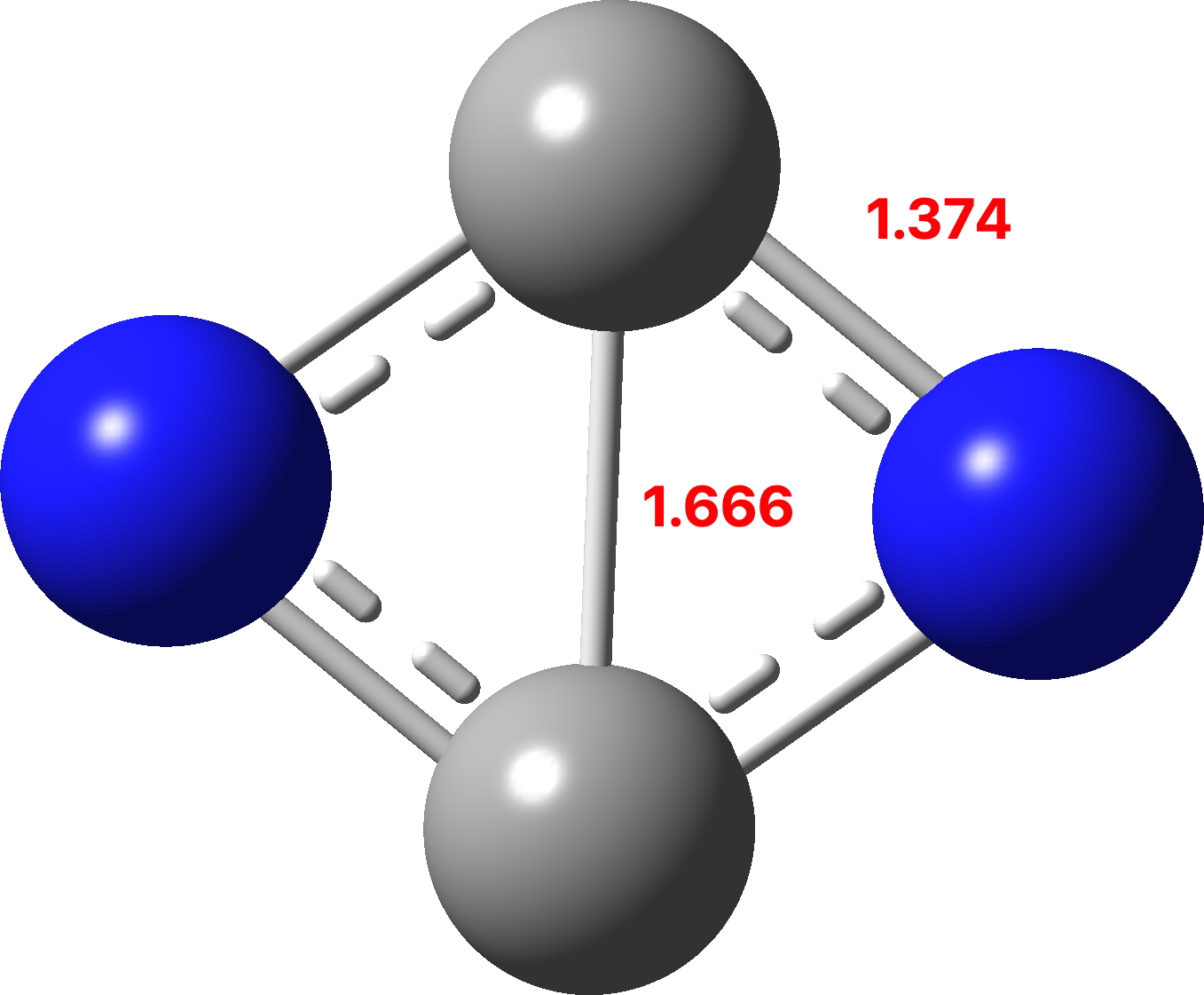
The previous examples of four atom systems displaying two layers of aromaticity illustrated how 4 (B 4 ), 8 (C 4 ) and 12 (N 4 ) valence electrons were partitioned into 4n+2 manifolds (respectively 2+2, 6+2 and 6+6). The triplet state molecule B 2 C 2 with 6 electrons partitioned into 2π and 4σ electrons, with the latter following Baird’s aromaticity rule.[1],[2]. Now for the final missing entry;

Respiratory pigments are metalloproteins that transport O 2 , the best known being the bright red/crimson coloured hemoglobin in human blood. The colour derives from Fe 2+ at the core of a tetraporphyrin ring. But less well known is blue blood , and here the colour derives from an oxyhemocyanin unit based on Cu 1+ (the de-oxy form is colourless) rather than iron.
The title comes from the abstract of an article[1] analysing why Biotin (vitamin B7) is such a strong and effective binder to proteins, with a free energy of (non-covalent) binding approaching 21 kcal/mol. The author argues that an accumulation of both CH-π and CH-O together with more classical hydrogen bonds and augmented by a sulfur centered hydrogen bond, oxyanion holes and water solvation, accounts for this large binding energy.

In my first post on this theme, an ELF (Electron localization function) analysis of the bonding in the molecule HO-S≡C-H (DOI: 10.1002/anie.200903969) was presented. This analysis identified a lone pair of electrons localized on the carbon (integrating in fact to almost exactly 2.0) in addition to electrons in the CC region.
I have long been fascinated by polymers of either carbon dioxide, † or carbon monoxide, or combinations of both. One such molecule, referred to as dioxane tetraketone when it was featured on the ACS molecule-of-the-week site and also known as the anhydride of oxalic acid, or more formally 1,4-dioxane-2,3,5,6-tetraone, has been speculated upon for more than a century.[1] The history of chemistry has many molecules whose
The quote of the post title comes from R. B. Woodward explaining the genesis of the discovery of what are now known as the Woodward-Hoffmann rules for pericyclic reactions.[1] I first wrote about this in 2012, noting that “*for (that) blog, I do not want to investigate the transition states”.* Here I take a closer look at this aspect. I will start by explaining my then reluctance to discuss transition states.
The science journal is generally acknowledged as first appearing around 1665 with the Philosophical Transactions of the Royal Society in London and (simultaneously) the French Academy of Sciences in Paris. By the turn of the millennium, around 10,000 science and medical journals were estimated to exist.
FAIR is one of those acronyms that spreads rapidly, acquires a life of its own and can mean many things to different groups. A two-day event has just been held in Amsterdam to bring some of those groups from the chemical sciences together to better understand FAIR. Here I note a few items that caught my attention. Fairsharing.org was the basis for several presentations.