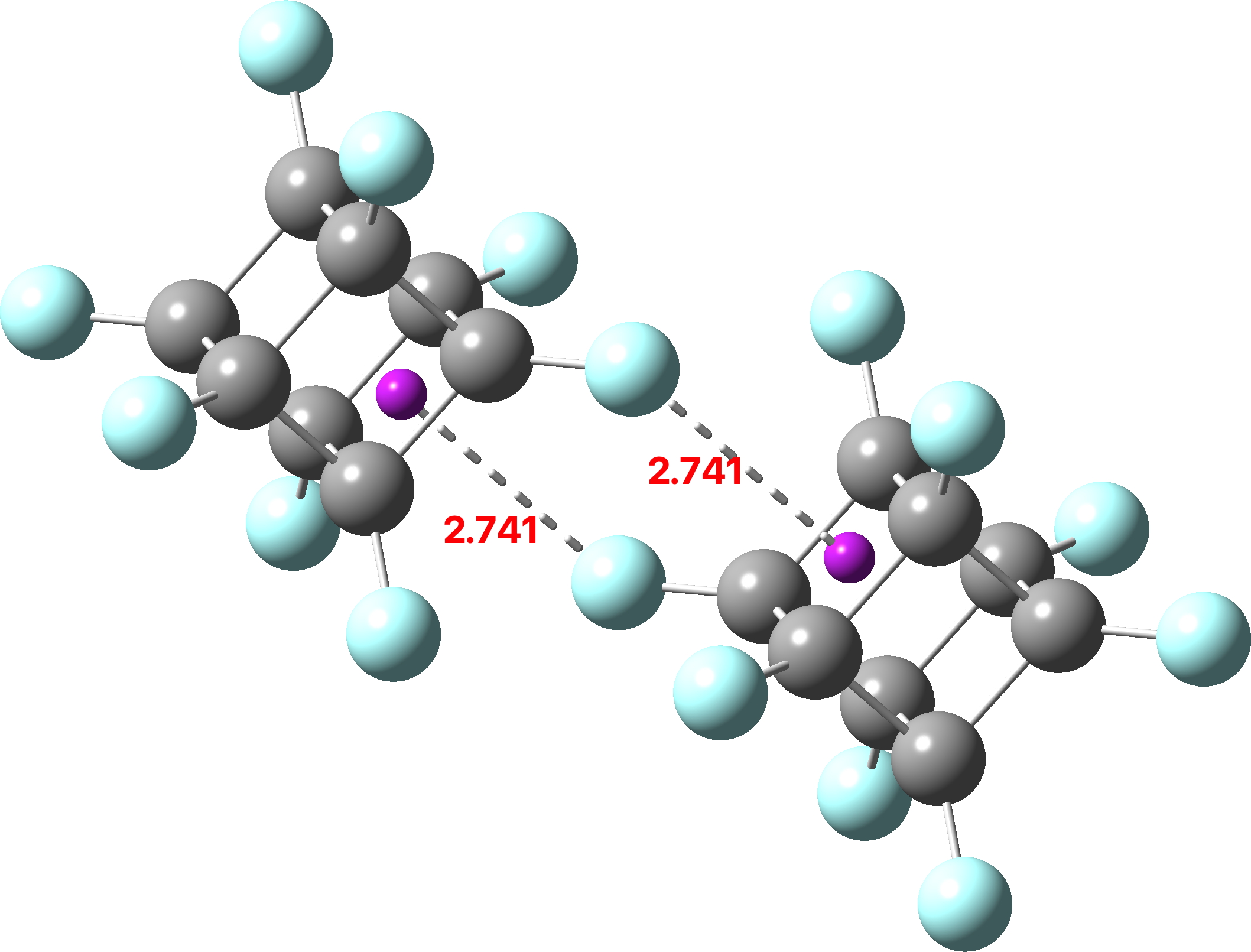
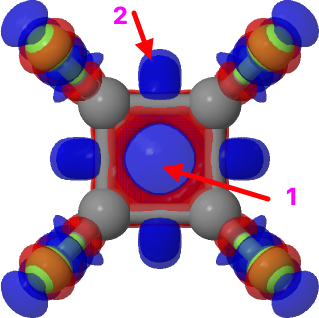
This is the second in what will hopefully become a series of blog posts (previously) focusing on the fascinating work of Dan Singleton (professor at Texas A&M). My goal is to provide concise and accessible summaries of his work and highlight conclusions relevant to the mechanistic or computational chemist. Today I want to discuss one of my favorite papers, a detailed study of the nitration of toluene by Singleton lab at Texas